



Metal adsorption in aqueous media using Moringa oleifera Lam. seeds produced in Ecuador as an alternative method for water treatment
Adsorción de metales en medio acuoso mediante semillas de Moringa oleifera Lam. producidas en Ecuador como método alternativo de tratamiento de aguas
ACI Avances en Ciencias e Ingenierías
Universidad San Francisco de Quito, Ecuador
Received: 17 November 2016
Accepted: 13 July 2018
Abstract: This work explores the technical viability in the use of Moringa oleifera Lam. seeds produced in Ecuador as an adsorbent medium for copper (Cu), nickel (Ni) and chromi- um (Cr) present in water. The seeds were prepared following a sequence of washing, drying, crushing, sieving, rewashing, and final drying. Two treatments were performed based on particle size. Treatment 1 consisted on a mixture of 70% of particles larger than 2 mm and 30% of particles between 1 and 2 mm; while Treatment 2 consisted only on 1 - 2 mm particles. Batch experiments were performed with metal concen- trations ranging from 10 to 150 ppm, a dose of 1.00 g of MO per liter, and mechanical stirring for 1 hour. Treatment 2 showed to be more favorable to metal removal and the Langmuir model better characterized adsorption of the three metals. The best kinetic description of the three metals is that of a pseudo first-order reaction where the ad- sorption capacities are 50.93 mg Cu/g MO, 30.14 mg Ni/g MO, and 40.98 mg Cr/g MO, with removal percentage of 37 - 53 %, 39 - 76%, and 11 - 33%, respectively.
Keywords: Moringa oleifera, isotherms, adsorption kinetics, metals, water treatment.
Resumen: Este trabajo presenta la viabilidad técnica de la utilización de semillas de moringa oleifera (MO) como medio adsorbente de cobre (Cu), níquel (Ni) y cromo (Cr) presente en agua. Las semillas siguieron un proceso de lavado, secado, triturado, tamizado, segundo lavado y secado final. Dos tratamientos en función de proporciones de tamaño de partículas fueron analizados. El primer tratamiento consistió en una mezcla del 70% de partículas con diámetros mayores a 2 mm y 30% de partículas con diámetros entre 1 y 2 mm; mientras que el segundo tratamiento consistió en partículas con tamaños entre 1 y 2 mm solamente. Soluciones de agua artificial de cada metal fueron preparadas con concentraciones de10 a 150 ppm y una dosificación de 1.00 g de MO fue añadida a cada litro de solución con un tiempo de agitación de 1 hora. El segundo tratamiento demostró ser más favorable a la remoción de metales. El modelo de Langmuir caracterizó la adsorción de los tres metales. La cinética que mejor describe la adsorción de los tres metales es de pseudo primer orden. Las capacidades de adsorción resultantes fueron: 50.93 mg Cu/g MO, 30.14 mg Ni/g MO, y 40.98 mg Cr/g MO, mientras que los rangos de remoción obtenidos fueron de 37 - 53 %, 39 - 76%, and 11 - 33%, respectivamente.
Palabras clave: Moringa oleifera, isotermas, cinética de adsorción, metales, tratamiento de agua.
INTRODUCTION
Industrial discharges, material degradation and anthropogenic activities are important sources of metal contamination in water bodies [1–4]. Since metallic contaminants tend to persist and accumulate in the food chain, they constitute a threat to human beings, animals and the surroundings. In order for these metals to be removed, a proper method of sequestration should be applied [2]. Traditional methods are based on adsorbing these contaminants on cationic compounds (such as aluminum sulfate) which are expensive and, sometimes, present deleterious effects in human health [5]. Therefore, a search for substitutes of these compounds and/or new technologies for metal removal in drinking water and wastewater treatment has directed the attention to new environmentally friendly technologies like Biosorption. Since this technique is easily adapted to the drinking water and wastewater treatment plants, it is thought to be the best option for a cost-effective solution.
Biosorption is a specific type of adsorption, characterized by the removal of contaminants (i.e. heavy metals in aqueous solution) by biological material through different mechanisms [3]. The process involves a solid phase, the bioadsorbent, and a liquid phase which contains the pollutant that need to be removed [4]. Bioadsorbent is the name given to any biological material possessing properties that confer high retention capabilities of a given pollutant, achieving the reduction of the concentration in the solution to a level of parts per billion [6].
In Ecuador, the advances in water resource recovery are very limited. For example, only 1% of wastewater is treated and it is discharged directly to water bodies. The first water resource recovery facility (WRRF) in the Quito Metropolitan District (the capital of Ecuador) started operation in March, 2017. This facility with wide coverage over the southern part of the city is an important milestone. Nevertheless, this plant is still insufficient to cover the needs of wastewater treatment of the city. Due to the high cost of chemicals for its operation, our research group is searching for a cost-effective alternative for future implementation at a pilot and real scale level.
Moringa oleifera Lam. (MO) from the Moringaceae family [6], depicted in Figure 1, is a crop native to South Asia that grows in the Himalayan foothills and is widely cultivated across the tropics and subtropics [7]. The leafs, seeds and pods are characterized by having good adsorptive properties and can be used as natural coagulants and flocculants, as well as filtration medium in water treatment [5,8-10]. Several studies [1,5,8,11-13]show that MO pods can remove organic pollutants and pesticides. MO seeds have good coagulant properties and are capable of removing organic and mineral particulates as well as heavy metals like lead, copper, cadmium, chromium and arsenic from water. Seed extracts have also antimicrobial properties [12]. Therefore, MO has become an environmental alternative to commonly used reagents (aluminum and ferric sulfate, and polymers) used in drinking and wastewater treatment [11,14,15] which have been proven to be more expensive than natural sorbents [1]. Depending on the soil conditions and other environmental variables, MO shows different chemical and nutrient compositions [12] that can affect its adsorptive, flocculant, coagulant and antimicrobial properties.

Moringa oleifera a) seeds, b) pods. Photographies taken by Jaime Cahuasquí.
In Ecuador, MO plantations exist in regions such as Santa Elena Peninsula and Pedernales- Manabí and is currently used for medicinal and dietary purposes [16,17]. Consequently, due to MO morphological characteristics and adsorption properties [1,5,8,12], it can be presented as a potential metal adsorbent substitute for chemicals used in drinking water and wastewater facilities in the Quito Metropolitan District, Ecuador. For this purpose, this research aims to evaluate the capability of MO produced in Ecuador to be used as metal adsorbent in water treatment applications.
The objectives of the present research involve: i) the use of Moringa oleifera produced in Ecuador (EMO) as an adsorbent medium, ii) the quantification of EMO effectivity in terms of copper, nickel and chromium removal, and iii) the gain of insightful information regarding the influence of EMO dose and particle size in metal adsorption capacity. Ongoing projects are being executed by our research group to explore the viability of EMO as coagulant, flocculant and filtering medium to remove suspended solids, turbidity, E. Coli, emerging contaminants, among other classic contaminants.
MATERIALS AND METHODS
Preparation of EMO
EMO seeds (Figure 1a) were collected and provided by Ecuamoringa (Guayaquil, EC090510, Ecuador). After reception, seeds were conditioned through six sequential steps: first washing, first drying, crushing, sieving, second washing, and final drying. Distilled water was used throughout washing operations. During the first stage of washing, dust and surface impurities were removed. Then, samples were allowed to dry at room temperature for 24 hours in a shadow area inside the laboratory. The first drying process was performed in an oven at 115 °C for 24 hours to remove volatile contaminants present in the seeds. A porcelain mortar was used to crush the EMO seeds and two samples were selected based on particle sizes. The first sample (Treatment 1) consisted of 70% of particles ≥ 2 mm in diameter, and 30% of particles 1-2 mm. During the initial stage of the project the most abundant fractions after the seeds were crushed in a mortar fell into these mesh sizes. The minor fraction (30%) passed the 2-mm sieve and was retained in the 1-mm sieve, while the larger fraction (70%) was retained in the 2-mm sieve. Treatment 1 consisted on the combination of these two fractions, and, in order to gain insight about particle size effects, Treatment 2 experiments used 100% of particles with diameters between 1 and 2 mm. The second washing cycle involved using distilled water until the water became clear. Finally, second drying was performed at 115° C for 24 hours.
Preparation of the metal containing solutions
Concentrated solutions containing 1000 ppm of copper (Cu) and nickel (Ni) were prepared by adding 3.925 g of CuSO4·5H2O and 4.050 g of NiCl2·6H2O to one liter of distilled water, respectively (Reagents HVO, Quito, Ecuador). Subsequently, a series of aqueous solutions with different concentrations of metals in the range of 10 to 150 ppm were prepared from the concentrated solutions. Regarding chromium (Cr), a 1000 ppm standard with 2% nitric acid AA13N-1 (AccusTrace, New Haven, CT 06513 USA) was used to prepare the solutions.
Adsorption experiments
In order to determine the effectiveness of the adsorbent and to understand the dynamics of the process, an investigation of isothermal adsorption can be performed. For this, two laboratory techniques could be used: batch (discontinuous process) and column (continuous process) experiments [18]. The batch method consists in applying constant agitation for different concentrations of the contaminant to study specific adsorbent doses. The adsorption capacity qe (mg metal ions adsorbed / g of adsorbent) is related to the equilibrium concentration of metal ions Ce (mg metal ions in the solution / L solution) [19] and this relationship is described by Equation 1:
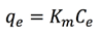 (1)
(1)where Km is the adsorption constant, or distribution coefficient, and the adsorption equilibrium capacity, qe, is given by Equation 2:
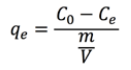 (2)
(2)where Co is the initial concentration of metal ions in aqueous solution (mg/L), m is the amount of dry adsorbent (g) and V is the volume of the aqueous solution (L).
The most widely used models describing adsorption mechanisms based on equilibrium relationships, adsorption kinetics, initial conditions, balance mass and energy of contaminants are [11]nickel, chromium and zinc ions from synthetic waste water by using Moringa aptera Gaertn (MAG: Langmuir [20], [21], Freundlich [22], [23] Temkin [24], [25] and Dubinin-Radushkevich [26], [27] which are explained briefly in the following sections.
Langmuir
This model describes a monomolecular adsorption without interaction between the adsorbed molecules with a finite number of active centers with the same energy [28]. This model is represented by Equation 3 [11]nickel, chromium and zinc ions from synthetic waste water by using Moringa aptera Gaertn (MAG:
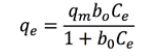 (3)
(3)where qm is the maximum adsorption capacity (mg of metal / g of adsorbent ion), b is the Langmuir constant (L solution / mg of metal ions), Ce is the equilibrium concentration of the dilution (mg ion metal in the solution / L solution).
Freundlich
The Freundlich model represents the relationship between a non-ideal and reversible adsorption [29]. It is applied to heterogeneous surfaces with interaction between the adsorbed molecules (multilayer). This model is described by Equation 4 [11]nickel, chromium and zinc ions from synthetic waste water by using Moringa aptera Gaertn (MAG):
 (4)
(4)where Kf (mg metal / g of solution ions) and n (dimensionless) are constants dependent upon the adsorbate and adsorbent at a particular temperature.
Temkin
This model is similar to the Langmuir model, but adds an adsorption energy condition which decreases linearly with increasing surface area of the adsorbent [24,25,30]. This model is represented by Equation 5 [11]nickel, chromium and zinc ions from synthetic waste water by using (MAG):
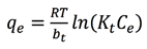 (5)
(5)where Kt is the Temkin constant (L solution / g of adsorbent), R is the ideal gas constant (8.314 J / mol K) and bt is a constant related to the heat of sorption (J / mol).
Dubinin-Radushkevich
This model proposes the theory of the volume filling of micropores (TVFM), being the micropores the most significant structures playing a role in adsorption, and it is based on the potential theory of adsorption introduced by Eucken and Polanyi [26,27,30-33]. This model is represented by Equation 6 [11]:
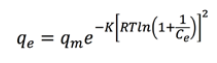 (6)
(6)where K is the constant of Dubinin-Radushkevich related to energy adsorption.
First and second order kinetics
The first order adsorption kinetics in a batch process involves assigning an active center for each metal ion, while the second-order kinetics involves two active centers for each metal ion [34]. These relationships can be represented by Equations 7 and 8, respectively [11]:
 (7)
(7)
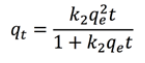 (8)
(8)where qt is the adsorption capacity according to the time, k1 and k2 are first and second order adsorption kinetic constants, respectively.
Isotherms
A batch process with continuous stirring was selected to study the adsorption capacity of EMO with respect to the metal ion concentration in equilibrium. The contact time for each solution (experiment) was set to 60 minutes, with a dose of 1.00 g of EMO per liter of solution.
Effects of EMO dose
The procedure explained previously was repeated, but different doses of adsorbent were selected: 0.25 g, 0.50 g and 1.00 g of EMO, to evaluate the adsorption capacity and determine whether this variation influenced the adsorption affinity already described.
Kinetics
Adsorption capacity studies with respect to time were executed to identify the behavior of metal adsorption dynamics. For this, ten experiments were performed over 100 ppm metal solutions (1 L) with 1.00 g of EMO at different contact time intervals. In each experiment, 10.0 mL aliquots were taken for subsequent analyses in an atomic absorption spectrometer (AAS). All experiments were performed in triplicate.
Analysis
The treated samples were analyzed with a Buck Scientific Atomic Absorption Spectrometer VGP 210 Model (BUCK SCIENTIFIC, East Norwalk, CT 06855, USA). An air/ acetylene flame and single-element hollow cathode lamps were used: Ni, Cr and Cu (BUCK SCIENTIFIC, East Norwalk, CT 06855, USA). The linear dynamic range according to the manufacturer for Cu, Ni and Cr are: 0.005-5 ppm, 0.2-4 ppm and 0.04-5 ppm, respectively.
Statistical Analysis
Cftool from Matlab® was used for obtaining the respective model parameters anddetermination coefficients. Other statistics such as the adjusted R2 value, SSE and RMSEwere also provided by Cftool and can be found as Supplementary Data.
RESULTS
Adsorption isotherms
The objective of obtaining the equilibrium concentration (Ce) was to calculate the adsorption capacity (qe) from Equation 2 to further express it in terms of the isotherm models already described and obtain the best correlation to the equilibrium curves.
Treatment 1
The model that best represent experimental metal adsorption was Langmuir for all three cases: Ni, Cu and C, as observed in Table 1. with coefficients of determination, R2, of 0.9769, 0.9982 and 0.9782, respectively. For comparison purposes, the R2 statistic is being shown throughout this work as it has been presented in similar studies [5]. The models predict the maximum adsorption capacity (qm), where Cu proved to be more easily adsorbed (qm =88.68 mg of metal ions/g of EMO). The removal percentage reached for Cu was 39%, while for Ni and Cr, a removal of 23% and 7%, were respectively achieved. Figure 2 shows the correlation of experimental data to isotherm models already mentioned, as described in Table 1. The cell with the highest R2 has been colored representing the best fit of experimental data to the proposed models.
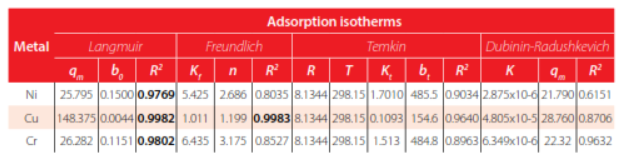
Model parameters – Treatment 1. The best model is represented by a colored cell based on the highest coefficient of determination, R2.
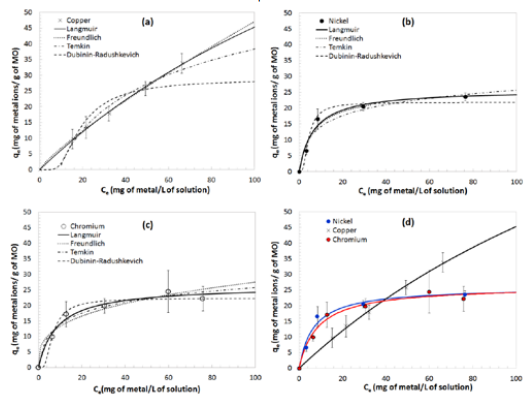
Treatment 1 - adsorption isotherms for a) Cu, b) Ni and c) Cr. A dose of 1.00 g MO into a 1 L solution, and a contact time of 1 h were used in each experiment. The best model is represented by a solid line. d) Presents a comparison of the three metals. The adsorption isotherm of Cr shown in Figure 2c used a different vertical axis scale to better visualize adsorption behavior.
Treatment 2
Similarly, for Treatment 2, Table 2 shows the model parameters obtained by fitting experimental data to the isotherm models. It can be observed that the experimental data of Cu and Ni maintain the previous models with R2 values equal to 0.985 and 0.976, respectively. In contrast, Cu experimental data was found to be best described by Langmuir with an R2 = 0.986. Figure 3 presents a graphical representation of these results, as well as a comparison between metals. Average percentage removals are 69% and 55% for Ni and Cu, while for Cr the average removal percentage is 31%.
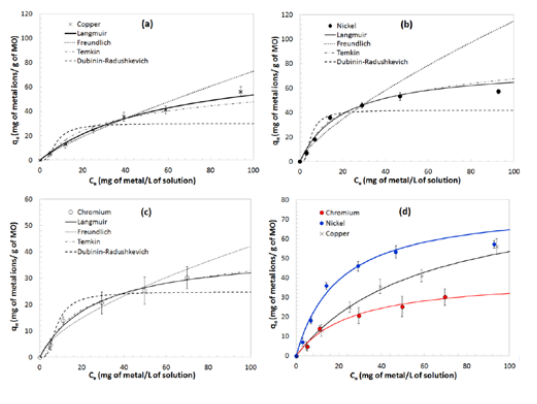
Treatment 2 - adsorption isotherms for a) Cu, b) Ni and c) Cr. A dose of 1.00 g MO into a 1 L solution, and a contact time of 1 h were used in each experiment. The best model is represented by a solid line. d) Presents a comparison of the three metals.
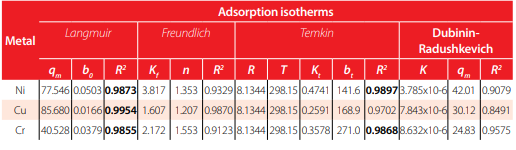
Model parameters – Treatment 2. The best model is represented by a colored cell based on the highest coefficient of determination, R2
Adsorbent dose
For the second stage of the study, Treatment 2 was used due to higher removal efficiencies preserving 1-hour contact time and 1 L of batch volume. Figure 4 presents the equilibrium concentration as a function of adsorbent dose for different initial metal concentrations. From Figures 4(a,c), for Cu and Ni, respectively, an increase in equilibrium concentration is observed when increasing initial concentrations of metal ions; additionally, there is a notable decrease in equilibrium concentrations when adsorbent dose increases from 0.5 to 1 g. Moreover, at lower initial concentrations (e.g. 10 ppm) the variability in equilibrium concentrations as a function of MO dose becomes less evident. Figure 4e (Cr), shows that there is a decreasing linear relationship between equilibrium concentrations as a function of increasing dose, even at low initial concentrations of metal ions. This implies that doses influence proportionally to the adsorption process of Cr.
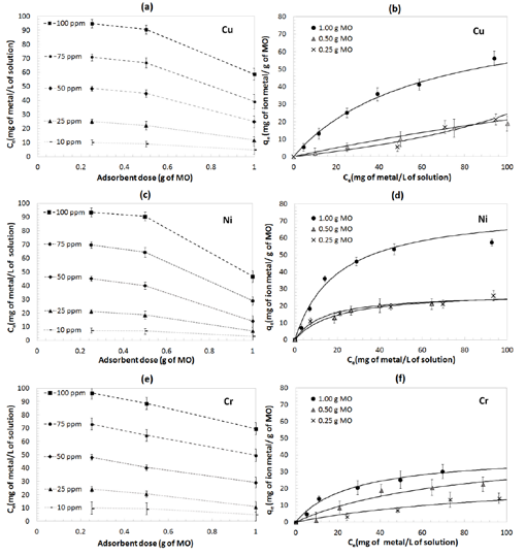
Effect of changes in MO dose on equilibrium concentration of metal ions (C ), for a) Cu, c) Ni e) Cr, and effect of dose on adsorption equilibrium (qe) for: b) Cu, d) Ni, f) Cr. Adsorbent doses of MO: 1.00 g, 0.50 g and 0.25 g in 1 liter of solution and a contact time of 1 hour.
Figure 4b represents the adsorbent’s dose influence over the adsorption isotherm of Cu; where at high concentrations of Cu, a greater adsorption of Cu at the equilibrium can be found. The adsorption capacity was altered significantly when the dose of 1.00 g of MO was reduced to 0.50 g of MO. Likewise, for Ni (Figure 4d) the same adsorption behavior is observed. The influence of MO dose on the adsorption process, especially at high initial concentrations, requires further investigation especially for Cu and Ni. Figure 4f shows a proportional variation of Cr adsorption isotherm regarding to a dose reduction of MO, therefore it is also possible to predict dose’s influence in Cr adsorption.
Contact time
A study of the dynamic behavior of the adsorption capacity (qt) with respect to time was performed until reaching an adsorption capacity of equilibrium or saturation state (qe). An initial concentration of 100 ppm of Cu, Ni and Cr and 1.00 g of MO (Treatment 2) was established. According to Figure 5, the time for the adsorption capacity (qe) for Cu, Ni, Cris 22, 18 and 28 min, respectively; being Ni, the metal that required the less contact time to reach a saturation point and greater removal efficiency. The best description of the three metals proved to follow a pseudo-first order kinetics with an R2 > 0.97, implying an active center MO adsorption for each metal ion.
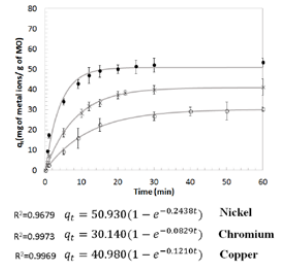
Experimental adsorption kinetics for 100 ppm of Cu, Ni and Cr. Kinetics is described by a pseudo first-order reaction, with R2 > 0.97.
Figure 5. Experimental adsorption kinetics for 100 ppm of Cu, Ni and Cr. Kinetics is described by a pseudo first-order reaction, with R2 > 0.97.
Comparison between metals
Table 3 focuses on the influence of the MO dose adjustment with respect to experimental adsorption data (i.e, the conservation of models). This table shows that the variation of MO dose did not alter the fit of the Langmuir model already identified for Ni, Cu and Cr, therefore, it is possible to predict adsorption behavior at lower and higher doses for further implementation in adsorbent systems.
| 1.0 g MO | 0.50 g MO | 0.25 g MO | |
| Ni | 0.9897/0.9873 | 0.9896 | 0.9832/0.9690 |
| Temkin/Langmuir | Langmuir | Freundlich/Langmuir | |
| Cu | 0.9954 | 0.9918 | 0.9714/0.9437 |
| Langmuir | Langmuir | Temkin/Langmuir | |
| Cr | 0.9868/0.9855 | 0.9664/0.9398 | 0.9279 |
| Temkin/Langmuir | Temkin/Langmuir | Langmuir |
Coefficients of determination (R2) for the best models characterizing experimental data with varying adsorbent dose (1.0, 0.50 and 0.25 g MO/L).
DISCUSSION
In the case of Treatment 1, it is presumed that the low Cr adsorption is influenced by the pH, wherein the initial solution of Cr has an acid pH (1.38) which is low compared to the pH of Cu (3.54) and Ni (5.48). A direct comparison is not recommended without having equal pH values, however it provides insights on the influence of pH over adsorption since a buffering capacity towards neutral conditions was observed as reported in previous studies [35-37].
In general, the determination coefficients, R2, exceeded a value of 0.970. Nevertheless, the removal of metals using Treatment 1 proved to be less than the removal obtained when Treatment 2 was used. For this reason, the rest of MO experiments used Treatment 2 for further analyses. It is presumed that better adsorption lies on the use of morehomogenous particle sizes (100% particles had diameters between 1 to 2 mm), and alsothe reduction of particle size allows a greater surface contact area which is essential formaintaining uniform contact during batch processes.
By comparing Figure 2c and Figure 3c, it appears that when Treatment 2 was used there is a greater adsorption capacity of Cr, while for Treatment 1 the equilibrium concentrations obtained for high initial concentrations (75 ppm onwards) are minimal; and this is another point that argues the importance of homogeneity of particles and their size. Despite the increase in the adsorption capacity (qe) of Cr, the results in Table 2 indicate that the adsorption capacity of Cu and Ni is 1.6 times larger than that of Cr. However further experimentation should be performed in order to fully understand theinfluence of pH on the adsorption isotherms and hence the removal efficiencies. Also, itis worth mentioning, as it was already done earlier in this manuscript, that it would notbe fair to make a direct comparison based on the results aforementioned since the pHvalues were different for each solution.
The methods and results obtained in this work can be compared, in some instances, with some important studies such as Matouq et al. [11] and García-Fayos et al. [13]. For example, Matouq et al., explored Cr, Cu, Ni and Zn adsorption using Moringa aptera Gaertn. pods. This work was the base study for the present study, however the present work tested for MO seeds. The paper by García-Fayos et al. [13] evaluates the efficiency of MO to remove heavy metals in aqueous solutions and similar essays were performed in terms of batch experiments, contact time, adsorbent dose, kinetic studies andadsorption isotherms. The selected metals for the analyses were Cd and Cu. Althoughthe methods could be compared, there are important differences. The present workuses the whole seed, which was then crushed, while the other study used MO huskonly which evidenced a 90% Cu removal. On the other hand, this work determined a Cu removal between 37 and 53%. García’s study correlates to Lagmuir’s model for Cuwhile this work to Freundlich. In addition to this, metal content was determined through FTIR whereas our work used AAS. It is our view that the separation of the husk form the cotyledon is impractical on-site (i.e. in a pilot plant or in a large scale plant).
Overall, the present work suggests working with “more uniform” particle sizes to obtain higher removal percentages. Nonetheless, our research group is working on determining particle size effects on contaminant removal through experimental design to improve the meaningfulness of particle size effect from a statistical point of view [39]. Also, as previously discussed the models presented are pH specific at initial conditions and there is certainly an influence on adsorption, as it affects the chemistry of solutions of metals, the activity of the functional groups of the adsorbent and competition of metal ions. Working with Cu and Ni salts resulted more favorable than working with a chromium standard that included a 2% nitric acid solution. This is supported by previous studies where an increase in metal adsorption with increasing pH was evidenced since both the proton and the metal ions compete for the same groups on the surface and thus affecting the surface charge and the overall attraction with the counter ions [1,37].
For perspective, a representative waste water discharge at the Metropolitan District of Quito (Ecuador) has an approximate pH of 5.40 [38], therefore Cu and Ni adsorption herein described provides insightful information, although multi-component experiments should be performed to better understand the selectivity of MO over the selected metals.
At last, a lot of research using Moringa has been performed in other countries, however, this is foreign to Ecuadorian reality. The contribution of this research seek to provide evidence of the adsorption capacity and behavior of metals over Moringa oleifera seeds available in Ecuador for substitution of commonly used chemicals or as a filtration medium.
The key criteria for this work were mainly the initial concentration of the pollutants in aqueous media, the adsorbent dose, particle size and proportions, and contact time. In general, Ni presented higher removal percentage, followed by Cu and Cr, with 39 - 76%, 37 - 53% and 11 - 33% respectively, when MO particles had sized between 1 and 2 mm.
Ongoing research is being held to understand the effects of other parameters that may influence the adsorption capacity of this biological material, such as particle size, pH, and the use of specific sections of MO seeds, including inner and outer layers and different combinations of those. Furthermore, the washing and drying processes of MO are being explored and their study is recommended; not only cleaning the surface impurities helps removing the oil from the MO, but freeing the pores is crucial for the adsorption mechanism to take place. A detailed examination using multicomponent water and from real sources is being performed including Cr, Cu, and Ni, and other metals and metalloids such as As, Cd, Pb and Fe. Also, once MO has served its purpose as an adsorbent medium for metal and contaminant removal, it is important to find methods to regenerate MO and recover the adsorbed metals. Next, experiments with real water sources will be decisive to propose the adequate contaminant removal technologies. These studies, along with emerging contaminants removal research are promising as it involves the application of new technologies friendly to the environment that could be incorporated into the future wastewater treatment plants in the Metropolitan District of Quito, and in any water, wastewater or industrial plant.
ACKNOWLEDGMENTS
This research was supported by the 2015 Collaboration Grant of the Universidad San Francisco de Quito-USFQ. Thanks the Programa para la Descontaminación de los Ríos de Quito (i.e. Quito’s Rivers Decontamination Program), especially, Dr.-Ing. Luis Antonio Gómez-Avila for supporting these research initiatives for future test and implementation in water resource recovery facility (WRRF) of the Quito Metropolitan District. Thanks to Dr. Egas from the Chemical Engineering Department-USFQ for his feedback and assistance during the development of this project.
AUTHOR’S CONTRIBUTIONS
This work was conceived by Andrea Landázuri. The funds were acquired and managed by Andrea Landázuri. The experimental methodology was planned by Jaime Cahuasquí and Andrea Landázuri. The experiments were performed by Jaime Cahuasquí and Andrés Lagos. The statistical analysis and curve fitting was executed by Andrea Landázuri. The tables and figures were produced by Andrea Landázuri and Jaime Cahuasquí. The interpretation of data was carried out by all the authors. All authors contributed to the manuscript.
REFERENCES
[1] Araújo, C. S., Alves, V. N., Rezende, H. C., Almeida, I. L., de Assunção, R. M., Tarley, C. R., ... & Coelho, N. M. M. (2010). Characterization and use of Moringa oleifera seeds as biosorbent for removing metal ions from aqueous effluents. Water Science and Technology, 62(9), 2198-2203.
[2] Volesky, B., & Holan, Z. R. (1995). Biosorption of heavy metals. Biotechnology progress, 11(3), 235-250.
[3] Ahalya, N., Ramachandra, T. V., & Kanamadi, R. D. (2003). Biosorption of heavy metals. Res. J. Chem. Environ, 7(4), 71-79.
[4] Izquierdo Sanchis, M. (2010). Eliminación de metales pesados en aguas mediante bioadsorción. Evaluación de materiales y modelación del proceso. Universitat de València.
[5] Martín, C., Martín, G., García, A., Fernández, T., Hernández, E., & Puls, J. (2013). Potenciales aplicaciones de Moringa oleifera. Una revisión crítica. Pastos y Forrajes, 36(2), 137-149.
[6] Volesky, B. (2001). Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy, 59(2- 3), 203-216.
[7] Food and Agriculture Organization of the United Nations. (2019). Moringa. Recuperado el 3 de julio de 2018 de: http://www.fao.org/traditional-crops/moringa/en/?amp%3Butm_medium=web&%3Butm_source=faohomepage.
[8] Ravikumar, K., & Sheeja, A. K. (2013). Fluoride removal from water using Moringa oleifera seed coagulation and double filtration. International Journal of Scientific & Engineering Research, 4(8).
[9] Shan, T. C., Al Matar, M., Makky, E. A., & Ali, E. N. (2017). The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Applied Water Science, 7(3), 1369-1376.
[10] Landázuri, A.C., et al.(2017). “Ewb-Ecuador/Usfq Project: Contaminant Removal from Effluents through the Use of Moringa Oleifera Seeds for Application in Ecuadorian Rural Communities,” in 2017 AIChE Annual Meeting Conference Proceedings.
[11] Matouq, M., Jildeh, N., Qtaishat, M., Hindiyeh, M., & Al Syouf, M. Q. (2015). The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. Journal of Environmental Chemical Engineering, 3(2), 775- 784..
[12] Gopalakrishnan, L., Doriya, K., & Kumar, D. S. (2016). Moringa oleifera: A review on nutritive importance and its medicinal application. Food Science and Human Wellness, 5(2), 49-56.
[13] Garcia-Fayos, B., Arnal, J. M., Piris, J., & Sancho, M. (2016). Valorization of Moringa oleifera seed husk as biosorbent: isotherm and kinetics studies to remove cadmium and copper from aqueous solutions. Desalination and Water Treatment, 57(48-49), 23382-23396.
[14] Garcia Fayos, B., ArnalArnal, J., & Alandia, S. (2012). Estudio de la descontaminación de efluentes líquidos con elevada concentración de metales pesados mediante bioadsorbentes de Moringa oleífera. In Instituto de Seguridad Industrial, Radiofísica y Medioambiental (ISIRYM). Universitat Politécnica de València. XVI Congreso Internacional de Ingeniería de Proyectos Valencia(pp. 11-13).
[15] Cardoso Valverde, K., Ferri Coldebella, P., Fernandes Silva, M., Nishi, L., Carvalho Bongiovani, M., & Bergamasco, R. (2018). Moringa oleifera Lam. and Its Potential Association with Aluminium Sulphate in the Process of Coagulation/ Flocculation and Sedimentation of Surface Water. International Journal of Chemical Engineering, 2018.
[16] Ecuamoringa.com. (2019). Ecuamoringa – Sembramos vida. Recuperado el 3 de julio de 2018 de: http://www. ecuamoringa.com/
[17] La moringa en Ecuador. (2015, mayo 17). El Universo. Recuperado el 3 de julio de 2018 de: http://www.larevista.ec/orientacion/salud/la-moringa-en-ecuador
[18] Souza, A. F., Câmara, L. D. T., & Neto, A. J. S. (2011). Modeling of Batch and Continuous Adsorption Systems by Kinetic Mechanisms. In Heat and Mass Transfer-Modeling and Simulation. InTech.
[19] Lo, S. F., Wang, S. Y., Tsai, M. J., & Lin, L. D. (2012). Adsorption capacity and removal efficiency of heavy metal ions by Moso and Ma bamboo activated carbons. Chemical Engineering Research and Design, 90(9), 1397-1406.
[20] Langmuir, I. (1916). The constitution and fundamental properties of solids and liquids. Part I. Solids. Journal of the American chemical society, 38(11), 2221-2295.
[21] Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical society, 40(9), 1361-1403.
[22] Freundlich, H.(1926). Colloid and Capillary Chemistry, 3rd ed. London: Methuen
[23] Friendly, H. (1930). Kapillare Chemie, Vol. 1. Leipzig: Akademische Verlagsgesellschaft.
[24] Temkin., M. I. and Phyzhev., W. M. (1940). Acta Physicochim. URSS, vol. 12.
[25] M. M. Dubinin and L. W. Radushkevich, Rend. Acad. Sci. URSS, vol. 55. 1947.
[26] Dubinin, M.M. (1952). Adsorption of gases and vapors and structure of adsorbents. Chemistry Advances, 21 (5), 513.
[27] Liu, S. (2016). Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design. Elsevier.
[28] Chen, X. (2015). Modeling of experimental adsorption isotherm data. Information, 6(1), 14-22.
[29] Eucken., A.(1914).Verhandlungen der Deutschen Physikalischen Gesellschaft, vol. 16.
[30] Dąbrowski, A. (2001). Adsorption—from theory to practice. Advances in colloid and interface science, 93(1-3), 135-224.
[31] Polanyi.,M.(1914).Verhandlungen der Deutschen Physikalischen Gesellschaft, vol. 16.
[32] Polanyi, M. (1916). Adsorption of gases (vapors) by a solid non-volatile adsorbent. Negotiations of the German Physekalische Gesellschaft, 18, 55-80.
[33] Pinzón-Bedoya, M. L., & Villamizar, L. E. V. (2009). Modelamiento de la cinética de bioadsorción de Cr (III) usando cáscara de naranja. Dyna, 76(160), 95-106.
[34] Aloo, B. N., & Yator, K. E. (2014). Effects of Moringa oleifera seeds on Escherichia coli, Enterobacter aerogenes, pH, and turbidity in water from selected sources in Kitale town.
[35] Olayemi, A. B., & Alabi, R. O. (1994). Studies on traditional water purification using Moringa oleifera seeds. African study monographs, 15(3), 135-142.
[36] Mangale, S. M., Chonde, S. G., Jadhav, A. S., & Raut, P. D. (2012). Study of Moringa oleifera (drumstick) seed as natural absorbent and antimicrobial agent for river water treatment. J Nat Prod Plant Resour, 2(1), 89-100.
[37] Senthilkumaar, S., Bharathi, S., Nithyanandhi, D., & Subburam, V. (2000). Biosorption of toxic heavy metals from aqueous solutions. Bioresource Technology, 75(2), 163-165.
[38] Landázuri, A. C., Quevedo, J. L., Torres, M. C., Mayorga, L. F., & Gómez-Ávila, L. A. (2014). Muestreo y Caracterización de la Descarga “Central Iñaquito”, Representativa de la Cuenca Urbana de la Quebrada El Batán: Quito-Ecuador. In Congreso Interamericano de Ingeniería Sanitaria y Ambiental (AIDIS) (pp. 1–12). Monterrey.
[39] Landázuri, A. C., Villarreal, J. S., Núñez, E. R., Pico, M. M., Lagos, A. S., Caviedes, M., & Espinosa, E. (2018). Experimental evaluation of crushed Moringa oleifera Lam. seeds and powder waste during coagulation-flocculation processes. Journal of Environmental Chemical Engineering, 6(4), 5443–5451.