



A comparison of small mammal communities in two High-Andean Polylepis woodlands in Ecuador Community of small mammals Polylepis Andean forest
Comparación de la comunidad de mamíferos pequeños en dos bosques alto andino de Polylepis, prístino y disturbado en Ecuador
ACI Avances en Ciencias e Ingenierías
Universidad San Francisco de Quito, Ecuador
Received: 10 August 2016
Accepted: 07 February 2019
Abstract: Polylepis forest, historically widespread throughout high elevations of the central and northern Andes, now remain only in discontinuous small patches. An expanding agricultural frontier, along with other anthropogenic pressures, imperils these remnants through further isolation and loss of habitat quality. Using two grids of live traps we compared the populations of small nonvolant mammals in an intact Polylepis woodland with one nearby that had been logged 50 years before. Our study is the first to examine the effects of habitat degradation and associated changes to vertical complexity and habitat heterogeneity on mammalian communities in Polylepis woodlands above 3500 m. The intact woodland had significantly more vertical complexity than the mid- successional woodland. A total of 315 captures of 147 individuals of 9 species were sampled during an intensive trapping effort in 2010. Trap success was especially high averaging 35.4% and 28.1% in the intact and mid-successional woodland, respectively. Diversity and abundance of small mammals were greater in the intact woodland than the mid-successional site. Thomasomys aureus forests specialist species were more abundant in the intact habitat; while Thomasomys paramorum, a habitat generalist, was dominant in both. Habitat quality affected movement patterns of T. paramorum. The results affirm a high diversity and density of small mammals in intact Polylepis woodland and indicate that the effects of habitat disturbance are species dependent. We suggest that habitat specialists are more susceptible to loss of habitat heterogeneity and vertical complexity than habitat generalists.
Keywords: Andes, habitat degradation, habitat heterogeneity, Polylepis woodland, succession.
Resumen: El bosque de Polylepis que históricamente estaba distribuido en altas elevaciones de los Andes centrales y del norte, ahora permanece sólo en pequeños parches discontinuos. La expansión de la frontera agrícola, junto con otras presiones antropogénicas pone en peligro los últimos remanentes a través de un mayor aislamiento y pérdida de la calidad del hábitat. Mediante el uso de dos rejillas con trampas vivas se compararon las poblaciones de mamíferos pequeños no voladores en un bosque de Polylepis prístino con uno cercano que había sido talado 50 años antes. El bosque prístino tenía complejidad vertical más significativa que el perturbado. Se obtuvieron en total 315 capturas de 147 individuos de 9 especies, durante un esfuerzo de trampeo en el año 2010. El éxito de captura fue alto, en promedio 35.4% y 28.1% en el bosque prístino y perturbado, respectivamente. La riqueza y abundancia de pequeños mamíferos fue mayor en el bosque prístino que en el perturbado. Thomasomys aureus un especialista de bosque fue más abundante en el hábitat prístino; mientras que T. paramorum un generalista de hábitat fue dominante en ambos. Los resultados revelan una alta diversidad y densidad de pequeños mamíferos en el bosque prístino de Polylepis, e indican que los efectos de la alteración de su hábitat podrían afectar a las especies dependientes. Sugerimos que las especies especialistas de hábitat son más susceptibles a la pérdida de la heterogeneidad y complejidad del hábitat vertical, que las generalistas de hábitat.
Palabras clave: Andes, degradación del hábitat, heterogeneidad del hábitat, bosque tropical altoandino, sucesión.
INTRODUCTION
The tropical Andes are biodiversity hotspots with high endemism and hence a region of conservation priority [1–2]. Among the most threatened tropical habitats are the high-andean tropical rain forests (sensu Grubb [3]) commonly referred to as Polylepis woodlands because their tree canopy is formed by virtually pure, monospecific stands of Polylepis [4]. Once historically widespread, these forests are now highly fragmented throughout the central and northern high Andes and often remain only along steep rock-strewn slopes and isolated ravines [5–6]. Interspersed among a matrix of alpine shrub-grassland known as páramo, the Polylepis woodlands in Venezuela, Colombia and northern Ecuador are geographically limited to elevations greater than 3000 m [7–8]. For centuries, anthropogenic factors such as fire, overgrazing, and timber extraction altered these geologically and climatically shaped landscapes [9]; but in Ecuador the agrarian reforms that began in the 1960’s accelerated the loss of old-growth Polylepis forests and páramo to agriculture [10] and have heightened the urgency for more ecological research. Not only do activities of people threaten regional biodiversity, but habitat loss and degradation affect vital ecological services, including the water resources for Andean cities [11], such as Bogota and Quito.
With the loss of old-growth tropical forests, the role of secondaryforests and those degraded by disturbances become potentially critical in maintaining biodiversity [12]. Yet, a complex array of spatial and temporal factors affects species composition and forest resilience. For instance, Chazdon and her colleagues [12] suggest that the proximity of remaining old-growth forests and the presence of seed-dispersing fauna influence the overall composition of / biotic communities in the secondary forests. The currently fragmented distribution of the Polylepis woodlands points to the vulnerability of this particular habitat to repeated human disturbances and the accessibility to people. Moreover, the remaining patches of woodlands that are closest to settlements and therefore most vulnerable to degradation are often biologically the more diverse because climatic conditions that are amenable to farming are also favorable to native plants and animals [13].
The ecotone between Polylepis forest and adjacent páramo is conspicuously abrupt. Cierjacks et al. [14], attribute the absence of a broad and gradual ecotone to the extreme climatic conditions and to the susceptibility of saplings and vegetative shoots to fire. They note that intact woodlands resist burning and are resilient to moderate grazing. The biotic response to fragmentation and degradation is species dependent. Pandit et al. [15], suggest that habitat specialists should be more susceptible to environmental factors, such as the heterogeneity and environmental conditions within a habitat, than habitat generalists. In turn, the overall landscape matrix of habitat patches more strongly influence the occurrence of habitat generalists than specialist species, which have narrow tolerance limits.
We sought to determine the effects of habitat degradation on the composition of small mammal communities in a Polylepis woodland and to examine heterogeneity of habitat structure, especially vertical complexity, as it affects mammalian species diversity and movement patterns. Because human disturbances increase environmental exposure and reduce forest structure and complexity of Polylepis woodlands [4], we predicted that habitat specialists would be more sensitive to habitat degradation than habitat generalists and that specialist diversity would be less in the mid-successional woodland than the intact woodland. Kirkland [16] and Fisher and Wilkinson [17], for example, have shown in temperate habitats that disturbances change the habitat structure and food resources that then alter small mammal communities, and species that are forest habitat specialists are especially vulnerable to changes in the vertical structure of habitat used for cover from predators and while foraging and nesting [18]. The present study is the first to attempt to delineate such effects in a high-andean tropical cloud forest.
Barnett [19] and Voss [20] described mammalian assemblages in Ecuador over long attitudinal gradients and across major habitat types (2700 m to 4000 m by Barnett and above 3000 m by Voss). Their studies note an extraordinarily high biodiversity of non-volant small mammals in Andean high-andean tropical cloud forest systems. Yet, despite their reports, few studies have examined the population ecology, survivorship, and habitat relationships of small mammals above 3 500 m in the Neotropics.
In order to assess the capacity of a mid-successional woodland to maintain biotic diversity and the implications for conservation, we chose to examine the mammals of Polylepis woodlands within one high Andean valley. Small mammals play a key role in community dynamics because they consume seeds and are also important in their dispersal [21,22]. We compared the structure of the communities of non-volant small mammals in an intact, mature homogenous Polylepis incana (Rosaceae) woodland with a nearby, disturbed mid-successional Polylepis woodland to determine the effects of habitat degradation.
We ascertained if movement patterns and habitat utilization were different for the two woodlands’ dominant species. Our study’s fieldwork, conducted in 2010, was augmented by data from 1,500 trap-nights in the intact woodland and adjacent páramo during June 2007, January 2008, and September 2008, which enabled us to determine habitat affinity, predict which species would be habitat generalists or specialists, and using recapture data, to ascertain survivorship in the intact woodland over an extended period.
MATERIALS AND METHODS
Study area
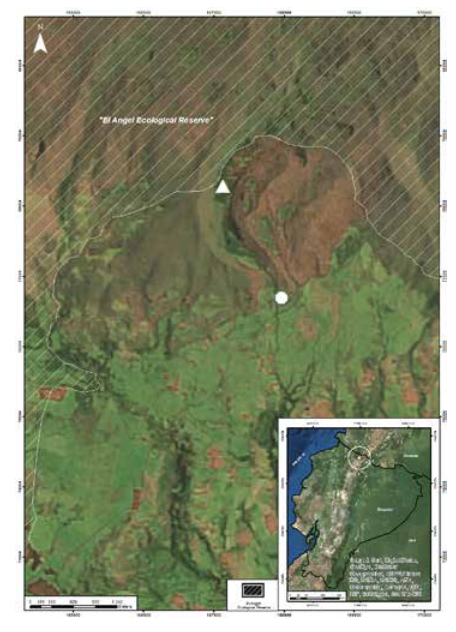
Location of the study site in northern Ecuador

Study site: A = Intact Polylepis woodland, B = Mid-successional Polylepis woodland
The study area is in and adjacent to El Angel Ecological Reserve of Carchi Province, Ecuador (Fig. 1). Field work was carried out at two 4-ha sites: a Polylepis dominant high andean tropical cloud forest (Fig. 2) and a mid-stage successional woodland from which Polylepis was logged approximately 50 years ago to obtain wood for building fences, making charcoal, heating and cooking. According to interviews with local land owners, for a few years following logging the more accessible portions of the site were partially maintained for pasturing cattle, and tree seedlings and woody vegetatives prouts were actively removed. The steep and ruggedly sloped banks of the river that divides the site retained mature trees. Use of the entire site stopped about 20 years ago when it was abandoned. The intact Polylepis woodland (0.714360°N, -77.982825°W, 3627 m) in the Ecological Reserve is located 1.2 km from the mid-successional woodland (0.703461°N,-77.976052°W, 3510 m). The Hondón River bisects both trapping sites and connects the two as a forested riverine corridor. The width of the corridor varies but at its narrowest is 30 m wide. Nearby, trees reach their upper elevational limit at 3700 m [23], and páramograsslands surrounds the woodlands and riverine corridors. The páramo is characterized by an abundance of Espeletia pycnophylla (Asteraceae) rosettes and tussock grasses. Annually the Reserve receives 1800 mm of precipitation with temperatures ranging between 18°C and 0°C [24]. Seasonality is determined more by rainfall than temperature. The three months of June, July and August are the driest with a monthly average of only 32 mm of precipitation. At the elevations of our study sites, temperature fluctuates greatly during any 24-hour period.
The intact woodland: was dominated by mature Polylepis incana that formed a dense, continuous, and tangled canopy 10 m high (Fig. 2A). Except for a few Oreopanax seemannianus (Araliaceae), an estimated 95% of the tree canopy was comprised of Polylepis. The average dbh of 20 randomly selected Polylepis was 37 cm with the greatest diameter being 60 cm. This forest was characterized by the visual dominance of Polylepis incana, and Oreopanax seemannium in lesser representation; the undergrowth housed shrubs such as Hypericum laricifolium, Weinmannia descendens, Gynoxys sodiroi, Brachyotum ledifolium, Myrsine andina and Valeriana microphylla, while the groundcover was composed ofnumerous herbaceous plants such as Galium hypocarpium, Rumex acetosella, Lachemillaorbiculata, Bromus pitensis, Polypodium monosorum and Oxalis Phaeotricha.
The mid-successional woodland: lacked a continuous canopy, and only about 5% of the area retained mature Polylepis trees. The presence of Polylepis stumps and accounts of local landowners indicated that the valley floor had been dominated by Polylepis about 60 years ago prior to logging. The remainder was a heterogeneous thicket of trees, shrubs and grasses. Approximately 15% of the mid-successional woodland was covered by the dominant shrub, Diplostephium floribundum (Asteraceae), which was more typically observed in the adjacent páramo than in mature forests (Fig. 2B). Individuals of this species reached a height of roughly 3 m.
Field survey
Live trapping occurred from 31 July to 12 August 2010, toward the end of the dry season. Traps were placed in a grid at 10-15 m intervals depending on habitat quality and slope. An effort was made to place traps near holes, runways and other sites of small mammal activity both in trees and on the ground. Traps were located with a Garmin C76 GPS unit to the nearest 3 m.
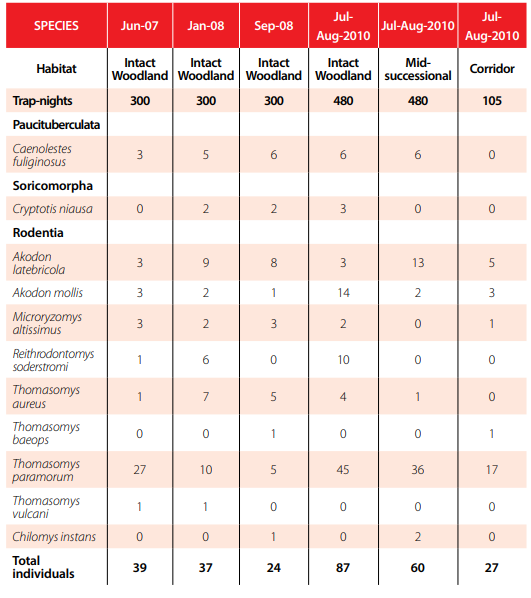
We trapped for three nights in the mid-successional woodland, moved the traps to the intact woodland for three nights and then repeated the trapping sessions for a total of 960 trap-nights. In addition, because we expected the riverine corridor that connected the mid-successional site to the intact woodland would ameliorate the potential loss of species or facilitate recovery following disturbances, we sampled the occurrence of mammals within the corridor with an effort of 105 trap-nights. Sherman live traps were baited with rolled oats and vanilla and checked each morning. Each animal was identified, measured, and marked with an ear tag and released at the point of capture as soon as possible. Procedures followed guidelines of the American Society of Mammalogists [25] and were approved by the Pacific Lutheran University Institutional Animal Care and Use Committee. Voucher specimens were deposited with Escuela Politécnica Nacional (MEPN), Quito.
To determine habitat heterogeneity and complexity, at each trap location we recorded three parameters, including size of the vertical and horizontal stems and the ground cover. The diameter at breast height (dbh) of the largest vertical stem within two meters of each trap was categorized as small (2-5 cm), medium (6-10 cm), or large (>11 cm). The diameter of the largest horizontal stem within two meters was also identified using the same size categories. For traps placed on the ground, we determined ground cover for a 0.5 m2 quadrat placed in front of the trap and evaluated the percent cover of bryophytes, forbs, woody debris, and bare ground. Each type was categorized as covering trace, 0-25 percent, 26-50 percent, 51-75 percent, or 76-100 percent of the quadrat. We also recorded the stratum of each trap reported as: (% of traps placed in stratum for intact/ mid-successional woodland). Traps were placed on the ground (6% /18%), understory 0.3-1 m (43%/16%), mid-story 1-2 m (29%/26%), or canopy >2 m (11%/51%). It was not possible to evenly distribute traps across the strata due to the limited availability of vertical structure in the mid-successional woodland.
Statistical analysis
The Mann-Whitney U-Test with a X2 approximation [26] was used to determine if the two habitats differed statistically in the size and abundance of vertical and horizontal stems, composition of ground cover, and the stratum or placement of traps. We used a Fisher’s Exact Test (FET) to determine if the abundance of each species differed between the two woodlands. We used FET due to the low numbers of individuals trapped per species. Ground cover did not show a significant difference between habitats, but the vertical complexity of the two habitats was distinct. The intact woodland had significantly more large vertical stems, (X21 = 38.004, P< 0.0001) and horizontal stems (X21 = 37.747, P < 0.0001). It also had more stratum levels or above-ground trap locations (X21 = 7.454, P = 0.0063).
RESULTS
Abundance and Diversity
Trap success at both sites was exceptionally high compared to lowland Neotropical sites [e. g., 27-29] with 35.4 percent trap success for the intact and 28.1 percent for the mid-successional woodland. A high trap success reflects relatively high densities of small mammals for both trapping grids, but the difference between the two suggests a depressed density at the mid-successional site. We captured 147 individuals of 9 species (Fig. 3). In the intact woodland there were 87 individuals of 8 species caught 180 times with 92 recaptures. The mid-successional site had 60 individuals of 6 species caught 135 times with 75 recaptures (Fig. 4). The Simpson Reciprocal Index of diversity was greater for the intact woodland (1/D = 1.62) than that of the mid-successional woodland (1/D = 1.22).
Habitat use
While the number of captures for each species was insufficient to associate with microhabitat parameters, we used a Fisher’s Exact Test (FET) to determine if the abundance of each species differed between the two woodlands. We used FET due to the low numbers of individuals trapped per species. Reithrodontomys soderstromi was not captured in the mid-successional woodland and therefore was more abundant in the intact woodland (Fig. 4; P = 0.0021). Neomicroxus latebricola was significantly more abundant in the mid-successional woodland (Fig. 4; P = 0.0091), while Akodon mollis was significantly more abundant in the intact woodland (Fig. 4; P = 0.0015). Microryzomys altissimus and Cryptotis niausa were only found in the intact woodland, while Chilomy sinstans was only in the mid-successional woodland, but in numbers too low to analyze statistically. Thomasomys paramorum was the most abundant species at both sites with 36 individuals captured in the mid-successional woodland and 45 in the intact woodland (Fig. 4); however, the abundance at the two sites was statistically similar (P = 0.1567). Only one T. aureus was captured in the mid-successional woodland, which precluded a statistical analysis; but it was more abundant in the intact woodland. A. mollis was caught not only on the ground and under woody debris, but occasionally on top of logs. Four species (T. paramorum, M. altissimus, A. mollis and N. latebricola) present at one of the two sites were also caught within the riverine corridor connecting the two sites. In addition, one T. baeops was captured in the corridor and was not found in eitherthe disturbed or intact woodland.

Small mammals registered in the area. A = Caenolestes fuliginosus, B = Cryptotis niausa, C = Akodon mollis, D = Chilomys instans, E = Neomicroxus latebricola, F = Microryzomys altissimus, G = Reithrodontomys soderstromi, H = Thomasomys aureus, I = T. paramorum.
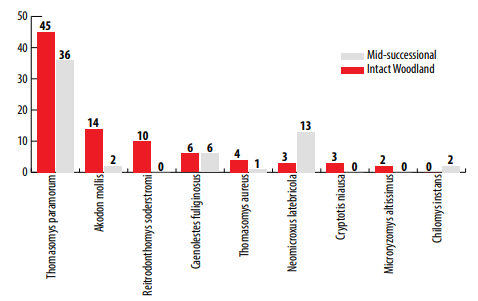
Abundance of small mammals in Intact and Mid-successional Polylepis woodland sites.
Movement
Mean distance moved between successive recaptures was calculated for T. paramorum. A total of 24 individuals in the mid-successional woodland and 25 in the intact woodlands were captured more than three times. In the intact woodland these individuals moved an average of 43 m, more than twice that of the mid-successional woodland (17 m). Our observations suggest that T. paramorum used the ground more frequently in both habitats; however, slightly less in the intact woodland than the mid-successional woodland, this result is influenced by differences in the availability of vertical habitat strata. In August 2010, we caught 5 of the 114 small mammals that had been captured, marked and released in the woodland in June 2007, January 2008 and September 2008 during a previous study. These recaptures revealed high longevity: one T. aureus male, one T. paramorum male, and one male R. soderstromi were at least three years old and another two T. paramorum (one male and one female) were likely four years of age, since when they were originally caught in 2007 they were the size typical of adults.
Natural history observations
In order to better understand resource partitioning, we recorded natural history observations, which have not previously been reported. One T. paramorum was seen eating fruits of Galium hypocarpium (Rubiaceae) and the spores of a bryophyte, and a T. aureus was observed chewing shoots of Dendrophorbium tipocochensis and Aetheolaena patens (Asteraceae). Caenolestes fuliginosus were typically caught near waterways, and this was especially pronounced in the mid-successional habitat that had substantial topographic relief near the stream. They were caught under and near large rocks and beneath the roots of Polylepis. One Caenolestes fuliginosus was tracked using the spool and line technique [30]. It stayed near the stream, moving close to the ground and using hollow stumps and dense vegetation. Small hairs were found in their feces, whichsuggests that C. fuliginosus feed on other small mammals.
DISCUSSION
Spatial heterogeneity affects the dynamics of small mammal populations and alters the community composition [31]. Large-scale land clearing creates a matrix of habitat patches potentially isolating populations; whereas degradation or small-scale, less intense disturbances promote a mosaic of successional stages affecting habitat quality. In already fragmented landscapes, such as high-andean tropical rainforests, the removal of a few key patches can substantially impact population viability [32]. As succession proceeds and vegetation changes, the communities of small mammals shift [33]. Viability and recovery of small mammal communities following disturbance are dependent on both the scale and intensity of the perturbation. As noted by Ricklefs [34], the occurrence of species within a matrix of habitats is dependent upon the capacity of individuals to disperse, survive, and reproduce. Food availability, habitat structure, and interspecific interactions within a habitat ultimately affect the distribution of the populations of each species.
Our study provides insights into the resilience and response of small mammals to habitat degradation and is the first to examine such effects in the high Andes. As revealed by the Simpson Reciprocal Index, diversity of the mammalian community was lower in the mid-successional woodland than in the intact one. While the mid-successional woodland had six species, only two less than the intact woodland; the two areas differed in composition, and the density of small mammals was lower in the trapping grid at the mid-successional woodland than the other. We expected that habitat specialists would be especially vulnerable to altered habitat quality. The mid-successional woodland had significantly less vertical habitat complexity than found in the intact woodland. Habitat structure likely affects food resource availability and hence the foraging characteristics and movements of small mammals. Indeed, T. paramorum moved more than twice as far between captures in the mid-successional woodland than in the one which was intact. Five decades after being logged, the mid-successional woodland site was a heterogeneous mix of trees, shrubs, grasses and forbs. It had yet to develop a closed canopy, which may have limited the presence of mammalian forest habitat specialists, such as T. aureus [20] and R. soderstromi (W. Teska, unpubl. data). These species may require more vertical structural complexity than was present in the mid-successional woodland. For instance, Brito et al. [35] found that T. aureus relies on a habitat’s vertical structure and complexity moving through trees and nesting above ground more frequently than T. paramorum. Temperatures within intact Polylepis woodlands have less diurnal variation and are less susceptible to frost than nearby páramo [14], which therefore opens the possibility that the microclimate, in addition to habitat complexity, may affect the viability of forest specialists outside of canopied forests. T. paramorum, a habitat generalist, [19, 35-37] was abundant in both the mid-successional and intact woodland. We predicted that two ground dwelling species, N. latebricola and A. molliswould occur in different abundances in the intact and mid-successional woodlands because N. latebricola was more associated with forested habitats than the páramo in this region [37]. Barnett [19] trapped A. mollis in a variety of habitats and considered it to be a habitat generalist. However in the present study, N. latebricola was statically moreabundant in the mid-successional woodland, and the reverse was the case for A. mollis. The distributional pattern of these two species suggests that vertical habitat complexity does not influence their occurrence.
It is noteworthy that the woodlands were connected by a forested riverine corridor that potentially augmented dispersal for some species and may account for the similar composition of mammals between the two sites. For example, T. aureus and R. soderstromi do not occur in adjacent páramo grasslands, but have been captured in the corridor in previous studies [37]. Corridors are known to facilitate dispersal and aid recovery in connected habitats, a species-dependent effect [38] that is important for forest habitat specialists [39]. Connectivity of the fragmented patches likely affects the resilience of the mammalian community.
High Andean Polylepis woodlands possess an exceptionally diverse and dense community of small mammals; yet, these cold woodlands are among the most endangered forest types in South America [14]. Small mammals and most notably those that are forest habitat specialists are susceptible to habitat degradation and to successional change of the woodlands. Because of the severe climatic conditions that typify elevations greater than 3500 m, recovery of forest structure following disturbance is slow [14]; and therefore, preservation of intact woodlands and maintenance of habitat corridors are vital to retaining their biodiversity. Our study points to the need to better understand the effects of human-induced disturbances in high-andean tropical cloud forests. More research is necessary to measure and evaluate the degree to which a species is a habitat generalist or specialist because the viability of populations is dependent upon structural heterogeneity of habitats and by the spatial matrix of habitats within the landscape.
ACKNOWLEDGEMENTS
Financial support was provided by the Wiancko Charitable Foundation and Pacific Lutheran University. R. Ojala-Barbour would like to thank a grant from the Fulbright U.S. Student Program for supporting data analysis. We thank Luis Albuja for institutional support on behalf of Escuela Politécnica Nacional, identification of voucher specimen, and guidance throughout the study. Carlos Cerón curator of the QAP of Universidad Central del Ecuador, Quito identified plant specimens. Fernando Acosta supported field logistics and the use of the Polylepis Lodge. Jesus Ortiz and Fernando Anaguano assisted in the field. Michael Behrens assisted with data analysis. Finally, we thank the Ministerio de Ambiente del Ecuador, MAE, for providing the research permission No. 002-08-IC-F-DRCI-MA.
AUTHOR’S CONTRIBUTIONS
WRT secured funding for this study and ran the analysis, ROB and JB drafted the manuscript; ROB and JB conducted fieldwork. All of the authors developed the study design and revised the manuscript.
REFERENCES
[1] Henderson, A., Churchill, S.P., & Luteyn, J.L. (1991). Neotropical plant diversity. Nature, 351:21–22. doi: http://dx.doi.org/10.1038/351021e0
[2] Myers, N., Mittermeier, R.A., Mittermeier, C.G., DAFonseca, G.A.B., &JKent. (2000). Biodiversity hotspots for conservation priorities. Nature, 403:853–858. doi: http://dx.doi.org/10.1038/35002501
[3] Grubb, P.J. (1977). Control of forest growth and distribution on wet tropical mountains: with special reference to mineral nutrition. Annual Review of Ecology Systematics, 8:83–107. doi: http://dx.doi.org/10.1146/annurev.es.08.110177.000503
[4] Toivonen, J.M., Kessler, M., Ruokolainen, K., & Hertel, D. (2011). Accessibility predicts structural variation of Andean Polylepis forests. Biodiverssity and Conservation, 20:1789–1802. doi: http://dx.doi.org/10.1007/s10531-011-0061-9
[5] Laegaard, S. (1992). Influence of fire in the grass páramo vegetation of Ecuador. In: Balslev H, Luteyn JL. (eds) Páramo. An Andean ecosystem under human influence. Academic Press, London, pp 151–170. Recuperado de http://documentacion.ideam.gov.co/cgi-bin/koha/opac-detail.pl?biblionumber=26903&shelfbrowse_itemnumber=28139
[6] Kessler, M. (2002). The “Polylepis Problem”: Where do we stand?. Ecotropica, 8:97–110. Recuperado de http://www.soctropecol.eu/publications/pdf/82/Kessler%20M%202002,%20Ecotropica%208_97-110.pdf
[7] Luteyn, J.L., & Churchill, S.P. (2000). Vegetation of the tropical Andes: an overview. In: Lentz DL (ed) An imperfect balance: landscape transformations in the Pre-Columbian Americas. Columbia University Press, New York, pp 281-310.
[8] Moscol-Olivera., M.C., & Cleff, A.M. (2009). A phytosociological study of the páramo along two altitudinal transects in El Carchi province, northern Ecuador. Phytocoenologia, 39:79-107. doi: http://dx.doi.org/10.1127/0340-269X/2009/0039-0079
[9] Sarmiento, F.O. (2002). Anthropogenic change in the landscapes of highland Ecuador. Geographical Review, 92:213-234. doi: http://dx.doi.org/10.1111/j.1931-0846.2002.tb00005.x
[10] López-Sandoval, M.F. (2004). Agricultural and Settlement Frontiers in the Topical Andes: The Páramo Belt of Northern Ecuador, 1960-1990. Institut fur geographie an der Universitat Regensburg Selbstverlag. Recuperado de https://bifea.revues.org/5553
[11] Luteyn, J.L. (1992). Páramos: Why study them?. In: Balslev H, Luteyn JL (eds). Páramo. An Andean ecosystem under human influence. Academic Press, London, pp 1–14.
[12] Chazdon, R.L., Peres, C.A., Dent, D., Sheil, D., Lugo, A.E., Lamb., D., Stork, N.E., & Miller, S.E. (2009). The potential for species conservation in tropical secondary forests. Conservation Biology, 23:1406–1417. doi: http://dx.doi.org/10.1111/j.1523-1739.2009.01338.x
[13] Fjeldsa, J. (2002). Polylepis forests – vestiges of a vanishing ecosystem in the Andes. Ecotropica, 8:111–123. Recuperado de http://www.soctropecol.eu/publications/pdf/8-2/Fjeldsa_J_2002_Ecotropica8_111-123.pdf
[14] Cierjacks, A., Wesche, K., & Hensen, I. (2007). Potential lateral expansion of Polylepis forest fragments in central Ecuador. Forest Ecology and Management, 242:477–486. doi: http://dx.doi.org/10.1016/j.foreco.2007.01.082
[15] Pandit, S.N., Kolasa, J., & Cottenie, K. (2009). Contrasts between habitat generalists and specialists: An empirical extension to the basic metacommunity framework. Ecology, 90:2253–2262. Recuperado de http://www.jstor.org/stable/25592741
[16] Kirkland, G.L. (1990). Patterns of initial small mammal community change after clearcutting of temperate North American forests. Oikos, 59:313–320. Recuperado de http://www.jstor.org/stable/3545141
[17] Fisher, J.T., & Wilkinson, L. (2005). The response of mammals to forest fire and timber harvest in the North American boreal forest. Mammal Review, 35:51–81. Recuperado de http://www.jasontfisher.ca/uploads/6/1/0/0/61006329/fisherandwilkinson2005.pdf
[18] Loberger, C.D, Theimer, T.C, Rosenstock, SS., & Wightman, C.S. (2011). Use of restoration-treated ponderosa pine forest by tassel-eared squirrels. Journal of Mammalogy, 92:1021–1027. doi: http://dx.doi.org/10.1644/10-MAMM-A-321.1
[19] Barnett, A.A. (1999). Small mammals of the Cajas Plateau, southern Ecuador: ecology and natural history. Bulletin of the Florida Museum of Natural History, 42:161–217. Recuperado de http://www.rebeccashapley.com/akodon/reprint_pdfs/99EcuadorLasCaJasSmallMammals.pdf
[20] Voss, R. (2003). A New Species of Thomasomys (Rodentia: Muridae) from eastern Ecuador, with remarks on mammalian diversity and biogeography in the Cordillera Oriental. American Museum Novitates, 3421:1–47. Recuperado de http://digitallibrary.amnh.org/bitstream/handle/2246/2850/N3421.pdfBodis?sequence=1
[21] Sahley, C.T., Cervantes, K., Pacheco, V., Salas, E., Paredes, D. & Alonso, A. (2015). Diet of a sigmodontine rodent assemblage in a Peruvian montane forest. Journal of Mammalogy 96: 1071–1080. doi: https://doi.org/10.1093/jmammal/gyv112
[22] Sahley, C.T., Cervantes, K., Salas, E., Paredes, D., Pacheco, V., & Alonso, A. (2016). Primary seed dispersal by a sigmodontine rodent assemblage in a Peruvian montane forest. Journal of Tropical Ecology, 32:125–134. doi: https://doi.org/10.1017/S0266467416000043
[23] Moscol-Olivera, M.C., & Cleff, A.M. (2009). Vegetation composition and altitudinal distribution of Andean rain forests in El Angel and Guandera reserves, northern Ecuador. Phytocoenologia, 39:175–204. doi: http://dx.doi.org/10.1127/0340-269X/2009/0039-0175
[24] INHAMI. unpubl. data. Meterological data for El Angel Ecological Reserve. Instituto Nacional de Metereología e Hidrología, Quito, Ecuador.
[25] Sikes, R.S., & Gannon, W.L. (2011). Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy, 92:235–253. doi: http://dx.doi.org/10.1644/10-MAMM-F-355.1
[26] Walpole, R.E., Meyers., R.H. Myers, S.L., &Ye, K. (1985). Probability and statistics for engineers and scientists. Macmillan Publishing, New York. Recuperado de http://imse.statler.wvu.edu/files/d/9656f528-f87e-4d33-94bf-1fb7c10f0e38/ieng213.pdf
[27] Woodman, N., Timm, R.M., SLADE, N.A., & Doonan, T.J. (1996). Comparison of traps and baits for censusing small mammals in Neotropical lowlands. Journal of Mammalogy, 77:274–281. Recuperado de https://kuscholarworks.ku.edu/bitstream/handle/1808/6927/Woodman%20et%20al.1996.pdf?sequence=1&isAllowed=y
[28] Tarifa, T., & Yensen, E. (2001). Mammals of Bolivian Polylepis woodlands. Revista Boliviana de Ecología y Conservación Ambiental, 9:29–44.
[29] López-Arévalo, H., Montenegro-Días, O., & Cadena, A. 1993. Ecología de los pequeños mamíferos de la Reserva Biológica Carpanta, en la Cordillera Oriental Colombiana. Studies on Neotropical Fauna and Environment, 28: (4) 193–210. doi: http://dx.doi.org/10.1080/01650529309360904
[30] Boonstra, R., & Craine, I.T.M. (1986). Natal nest location and small mammal tracking with a spool and line technique. Cannadian Journal of Zoology, 64:1034–1036. Recuperado de https://tspace.library.utoronto.ca/bitstream/1807/445/2/Tracking_with_spool.pdf
[31] Tews, J., Brose, U., Grimm, V., Tielbörger, K., Wichmann., M.C, Schwager, M, & Jeltsch, F. (2004). Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. Journal of Biogeography, 31:79-92. doi: http://dx.doi.org/10.1046/j.0305-0270.2003.00994.x
[32] Purcell, J., & Brelsford, A. (2004). Reassessing the causes of decline of Polylepis, a tropical subalpine forest. Ecotropica, 10:155-158. Recuperado de http://www.soctropecol.eu/publications/pdf/10-2/Purcell,Brelsford%202004.pdf
[33] Fox, B.J., Taylor, J.E., & Thompson, P.T. (2003). Experimental manipulation of habitat structure: a retrogression of the small mammal succession. Journal of Animal Ecology, 72:927–940. doi: http://dx.doi.org/10.1046/j.1365-2656.2003.00765.x
[34] Ricklefs, R.E. (2008). Disintegration of the ecological community. American Naturalist, 172:74–750. Recuperado de http://www.jstor.org/stable/10.1086/593002
[35] Brito, J., Teska, W.R., & Ojala-Barbour, R. (2012). Descripción del nido de dos especies de Thomasomys (Cricetidae) en un bosque alto andino en Ecuador. Therya, 3:263–268. doi: http://dx.doi.org/10.12933/therya-12-71
[36] Pacheco, V. (2015). Genus Thomasomys Coues, 1884. Pp: 617–682, in: Mammals of South America. Volume 2, Rodents. (JL Patton, UFJ PARDIÑAS y G D’ELÍA, eds.). The University of Chicago Press. Chicago, Estados Unidos. Recuperado de http://press.uchicago.edu/ucp/books/book/chicago/M/bo18553844.html
[37] Brito, J, Teska, W.R., & Ojala-Barbour, R. (2015). Guía de los pequeños mamíferos terrestres del bosque de Polylepis y Páramo de Frailejón del norte de Ecuador. Serie de Publicaciones del Museo Ecuatoriano de Ciencias Naturales del Instituto Nacional de Biodiversidad. INB-MECN. Publicación Patrimonio Natural del Ecuador Nro 3. Quito, Ecuador. doi: http://dx.doi.org/10.13140/RG.2.1.2400.6483
[38] Mabry, K.E., Dreelin, E.A., & Barrett, G.W. (2003). Influence of landscape elements on population densities and habitat use of three small-mammal species. Journal of Mammalogy, 84:20–25. doi: http://dx.doi.org/10.1644/1545-1542(2003)084<0020:IOLEOP>2.0.CO;2
[39] Mech, S.G., & Hallett, J.G. (2001). Evaluating the effectiveness of corridors: a genetic approach. Conservation Biology, 15:467-474. doi: http://dx.doi.org/10.1046/j.1523-1739.2001.015002467.x
APPENDIX 1
Specimens of reference
Cryptotis niausa: MEPN 10624-25, 10695; Caenolestes fuliginosus: MEPN 10628, 10888; Akodon mollis: MEPN 10491, 10643, 10772; Chilomys instans: MEPN 10875; Microryzomys altissimus: MEPN 10483, 10489-90; Neomicroxus latebricola: MEPN 10869-70, 10882, 10886-87; Thomasomys aureus: MEPN 10486, 10685-56; Thomasomys baeops: MEPN 10883; Thomasomys paramorum: MEPN 10492, 10493, 10880; Thomasomys vulcani: MEPN 10487, 10647; Reithrodontomys soderstromi: MEPN 10488.
Frequency of capture by species during each trapping session