Sección B: Ciencias Biológicas y Ambientales
Host species of the hemiparasitic shrub Phoradendron nervosum Oliv. in densely urban areas of Quito, Ecuador
Especies hospederas del arbusto hemiparasítico Phoradendron nervosum Oliv. en zonas urbanas de Quito, Ecuador
ACI Avances en Ciencias e Ingenierías
Universidad San Francisco de Quito, Ecuador
ISSN: 1390-5384
ISSN-e: 2528-7788
Periodicity: Bianual
vol. 15, no. 2, 2023
Received: 06 November 2023
Accepted: 29 November 2023

This work is licensed under Creative Commons Attribution-NonCommercial 4.0 International.
Abstract: Phoradendron nervosum is a hemiparasitic shrub with a wide distribution. Despite this, the ecology and natural history of this species is not well studied. Host species of P. nervosum are reported from Mexico and Costa Rica, with recent reports from Ecuador. Inside the urban settings of the metropolitan area of the city of Quito, we found during our sampling that P. nervosum infects 21 species of plants. The most common infected species were introduced and cultivated species, which appear to be more prone to infection than native species, native and cultivated species, and introduced species. Through this study, the number of host species of P. nervosum reported in Ecuador increases from 11 species, reported in previous studies, to 27 species. More sampling is needed in other areas in the wide distribution of P. nervosum to test if introduced and cultivated species are more prone to infection than the other infected species categories, and to expand the list of the host species of this common species.
Keywords: Parasitic plants, natural history, Viscaceae, urban ecology.
Resumen: Phoradendron nervosum es un arbusto hemiparásito con una amplia área de distribución. A pesar de ello, la ecología y la historia natural de esta especie no está bien estudiada. Se reportan especies hospedantes de P. nervosum en México y Costa Rica, principalmente con informes recientes de Ecuador. En nuestro muestreo dentro de las áreas urbanas del área metropolitana de la ciudad de Quito, encontramos que P. nervosum infecta 21 especies de plantas. Las especies infectadas más comunes eran especies introducidas y cultivadas las cuales son más propensas a la infección en comparación con especies nativas, especies nativas cultivadas y especies introducidas. Este estudio eleva las especies hospederas reportadas en estudios previos de P. nervosum en Ecuador de 11 especies a 27 especies incluidas las especies reportadas en nuestro estudio. Se necesitan más muestreos en otras áreas de la amplia distribución de P. nervosum para probar si las especies introducidas y cultivadas son más propensas a la infección que las otras categorías de especies infectadas y también para aumentar la lista de especies hospederas de esta especie común.
Palabras clave: Plantas parásitas, historia natural, Viscaceae, ecología urbana.
Phoradendron nervosum, colloquially referred to as “Matapalo,” “Muérdago,” or “Injerto” in South and Central America [1], is a hemiparasitic shrub that infects stems, a typical characteristic of the Phoradendron genus [2]. Despite the extensive native distribution of P. nervosum, which extends from Mexico to Bolivia, research about its natural history and ecology is limited. Particularly, studies related to host specificity have been performed mainly in Mexico and Costa Rica, with recent observations from Ecuador (Table 1) [1-12].
| Country | Species | Family | Reference |
| Ecuador | Prunus serotina | Rosaceae | [11] |
| Populus deltoides | Salicaceae | ||
| Acacia melanoxylon | Fabaceae | ||
| Acacia dealbata | Fabaceae | ||
| Salix humboldtiana | Salicaceae | ||
| Hibiscus rosa-sinensis | Malvaceae | ||
| Mimosa quitensis | Fabaceae | [12] | |
| Populus dealbata | Salicaceae | ||
| Callistemon citrinus | Rutaceae | ||
| Nerium oleander | Apocynaceae | ||
| Iochroma cyaneum | Solanaceae | ||
| Mexico (Querétaro) | Bumelia spp | Sapotaceae | [1] |
| Heliocarpus spp | Tiliaceae | ||
| Mexico (Veracruz) | Croton xalapensis | Euphorbiaceae | [8] |
| Heliocarpus sp | Tiliaceae | ||
| Ageratina ligustrina | Asteraceae | ||
| Lippia myriocephala | Verbenaceae | ||
| Mexico (Chapulhuacán) | Melia sp | Meliaceae | [9] |
| Mexico (Bajío) | Bumelia spp | Sapotaceae | [10] |
| Heliocarpus spp | Tiliaceae | ||
| Melia sp | Meliaceae | ||
| Costa Rica (Puerto Jimenez) | Synclairia polyantha | Asteraceae | [2] |
| Souroubea sp | Marcgraviaceae |
The objective of this study is to expand the list of reported host species of P. nervosum within urban environments in Quito, the capital of Ecuador. Existing literature and preliminary field observations demonstrate that P. nervosum is parasitizing a wide range of plants including native, introduced, and cultivated species within Quito [11,12]. Due to its wide distribution, we assume that P. nervosum infects native, introduced, and cultivated species in similar frequencies. This information holds the potential to enhance our understanding of the ecological dynamics and natural history of this widely distributed species within urban settings across the Americas.
The city of Quito is located in the central-northern part of the Inter Andean region of Ecuador, at an average of 2850 m.a.s.l. Its valleys sit on the hillside of numerous volcanos, creating a variety of climatic floors and plant formations, including piedmont rainforest, high Andean scrub forest, dry forest, and others [13]. This study was performed across urban and densely populated areas in north and southern Quito, including the adjacent valleys of Los Chillos, Tumbaco, and Pomasqui, which are now part of the urban area of Quito [14]. The sampling limit to the north was San Antonio de Pichincha, and to the south was Chillogallo neighborhood. The eastern limits were Tumbaco and Los Chillos valleys, and the western limits were the Guagua Pichincha and Atacazo volcanoes. For the most part, these urbanized localities harbor small green areas that consist of gardens, parks, and abandoned land, which have some native vegetation, but most of the coverage consists of ornamental species and invasive vegetation.
Sampling was performed by three researchers between May and December of 2019, traveling by car in equidistant transects along and around eight principal roads inside our study area. We sampled a total distance of 120 km. The sampled roads were Antonio José de Sucre Avenue, Simon Bolivar Highway, Rumiñahui Highway, Manuel Cordova Galarza Avenue, Antonio José de Sucre Avenue, Ruta Viva Highway, 6 de Diciembre Avenue, and 10 de Agosto Avenue.
P. nervosum is easy to recognize due to its oblong or lanceolate leaves that contrast with those of the host tree, often presenting a conspicuous yellowish color in the stems. P. parietarioides also occurs in our study area and is similar in morphology when compared to P. nervosum but easily distinguished by a greyish coloration [11,12]. Each time we recognized an infested tree, we approached it to identify the host species and took a photograph. We registered the frequency of infected individuals of each species, and to avoid pseudo replication we marked each host individual. Additionally, removing parasitic plants may disrupt the co-evolutionary dynamics between the parasites and their host species, also removing the transmission of adaptive information for successful infection in their seeds [15]. In this context, no collections were made during this study; all records and species identification were photographic [16]. For some species, photographs were not possible to take because the infected trees were growing on private land and access was not granted.
We identified each host species using samples from the “Herbario de Botánica Económica de la Universidad San Francisco de Quito (QUSF)” and classified them into four categories: native, introduced, introduced and cultivated, and native and cultivated. These categories are based on the growing status of this species in Ecuador, which is compiled in the Catalog of the Vascular Plants of Ecuador [17]. To test if one of these categories was more prone to infection by P. nervosum, we performed a chi-square goodness-of-fit test including the standardized residuals as post-hoc analysis using R statistical analysis software version 4.1.1 [18]. The relative frequency of each species was calculated.

FIGURE 1-a
Photos of P. nervosum host species. A. Phoradendron nervosum. B. Acacia baileyana. C. Acacia macracantha. D. Acacia mearnsii. E. Annona cherimola. F. Callistemon subulatus. G. Croton wagneri. H. Ficus carica. I. Hibiscus rosa-sinensis. J. Nerium oleander. K. Nicotiana glauca. L. Prunus persica.
Photos by Martín Carrera, Lía Altamirano, and Karla Barragán.
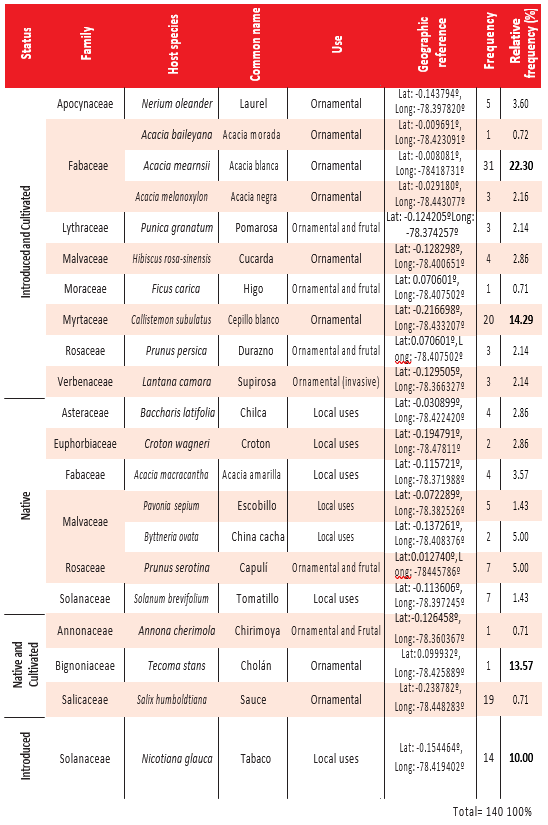
P. nervosum appears to be a generalist hemiparasite plant, infecting 21 species from 14 families from the Eudicotyledonae class. The family with more frequently infected species is Fabaceae (4 species), followed by Malvaceae (3 species) and Solanaceae and Rosaceae (each with 2 species). In families like Apocynaceae, Asteraceae, Bignoniaceae, Lythraceae, Myrtaceae, Salicaceae, and Verbenaceae, we only found one infected species (Fig 1 a-b). From the 140 infected individuals reported, the four species with highest relative frequency are Acacia mearnsii with 22.30 %, followed by Callistemon subulatus with 14.29 %, Salix humboldtiana with 13.57 %, and Nicotiana glauca with 10.00 %. We registered 3 infected species with only one individual (Tecoma stans, Acacia baileyana, and Annona cherimola, each with 0.71 %) (Table 2).
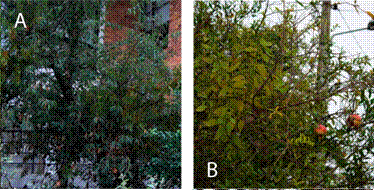
FIGURE 1-b
Photos of P. nervosum host species. A. Prunus serotina. B. Punica granatum. C. Salix humboldtiana. D. Solanum brevifolium.
Photos by Martín Carrera, Lía Altamirano, and Karla Barragán.
The chi-square goodness-of-fit test suggests that not all the categories have the same probability of infection (X2 = 9.2857, df = 3, . = <0.05). The category with higher standardized residuals is introduced and cultivated (2.0730), suggesting that this category is more prone to infection by P. nervosum. From the four categories in Figure 2, 53.06% of the identified infected individuals correspond to the introduced and cultivated category (10 species), 22.15% to native species (7 species), 14.99% to native and cultivated species (3 species), and 10% to introduced species (1 species) (Fig. 2).
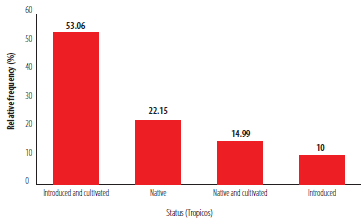
Figure 2
Relative frequency of the individuals infected by Phoradendron nervosum represented by their respective status according to Tropicos Introduced and Cultivated Native Native and Cultivated and Introduced
Our results increase the host species list of P. nervosum in Ecuador from 11 species reported in the literature to 27 species, contributing to the knowledge of the natural history and ecology of this species [11-12]. Despite being a relatively abundant species in Quito, studies of P. nervosum in Ecuador are scarce. Further sampling in areas within its natural distribution could give us a better understanding on the abundance and preferences of parasitizing of these species.
This research suggests that most of the infected individuals are introduced and cultivated species, mainly ornamental plants from gardens. The most logical explanation is that introduced and cultivated individuals are more prone to infection just because they are more abundant in urban areas such as Quito. Complementary to this explanation, ornamental plants often receive abundant water and nutrient provisioning, from which parasitic plants could also benefit and spread onto surrounding individuals. Another possible explanation is that introduced species have not co-evolved with P. nervosus, and therefore, they lack defense mechanisms against this parasitic species’ infection strategies [15]. These hypotheses open new doors for research on the ecology and infection mechanism of P. nervosum in urban areas.
Personal observations suggest that P. nervosum prefers dryer areas (e.g., dry forest) within Quito, especially around the north of Quito and Tumbaco Valley [19]. In the south of Quito and Los Chillos Valley, which are more humid areas, individuals were harder to find. These observations create new research questions about P. nervosum environmental preferences and biogeography.
Acknowledgments
We would like to thank Vlastimil Zak and Pablo Riera (QUSF) for useful comments about the host species of P. nervosum and for allowing us to check QUSF collections. We are grateful to the several landowners who allowed us to take photos of P. nervosum on their land. This research was funded by the authors exclusively.
REFERENCES
[1] Rzedowski, J. y Rzedowski, G. (2011 a). Principales hospederos y algunos otros datos ecológicos de las especies de Viscaceae en el estado de Querétaro. Instituto de ecología, Centro regional del Bajío.
[2] Morales, J. F. (2015). Manual de plantas de Costa Rica. Vol. VIII. Monogr. Syst. Bot. Missouri Bot. Gard., 131, 13-36.
[3] Kuijt, J. (2003). Monograph of Phoradendron (Viscaceae). American society of plant taxonomists. 66, 1-643. doi: https:// doi.org/10.2307/25011253
[4] Lobo, S. (2003). Los hospederos de las plantas hemiparásitas de la familia Loranthaceae (S.L.) en Costa Rica. Lankesteriana, 6, 17-20. doi: https://doi.org/10.15517/lank.v3i1.23083
[5] Espejel, I., Jiménez-Orocio, O., Castillo-Campos, G., Garcillán, P. P., Álvarez, L., Castillo-Argüero, S., Durán, R., Ferrer, M., Infante-Mata, D., Iriarte, S., León de la Luz, J. L., López-Rosas, H., Medel Narváez, A., Monroy, R., Moreno-Casasola, P., Rebman, J. P., Rodríguez-Revelo, N., Sánchez-Escalante, J. y Vanderplank, S. (2017). Flora en playas y dunas costeras de México. Acta Bot.nica Mexicana, 121, 39-81.
[6] Martínez, M., Evangelista, V., Basurto, F., Mendoza, M. y Cruz-Rivas, A. (2007). Flora útil de los cafetales en la Sierra Norte de Puebla, México. Revista Mexicana de Biodiversidad, 78, 15-40.
[7] Alvarado-Cárdenas, L. (2010). Flora del valle de Tehuacán-Cuicatlán. Instituto de Biología, Universidad Nacional Autónoma de México.
[8] Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (Conabio). (2011). La biodiversidad en Veracruz: Estudio de Estado. Instituto de Ecología, Universidad Veracruzana.
[9] Cibrián, D. y Cibrián, V. (2011). Distribución ecológica y propuesta de manejo integrado de muérdago Phoradendron falcifer del Suchiate (Liquidambar macrophylla) en el bosque mesófilo de montaña de la Sierra Alta del estado de Hidalgo. Innovando juntos, 7, 4-13.
[10] Rzedowski, J. y Rzedowski, G. (2011 b). Viscaceae. Instituto de ecología, Centro regional del Bajío.
[11] Cerón-Martinez, C. E. y Reyes Tello, C. I. (2022). Una planta hemiparásita muy agresiva en el campus de la Universidad Central del Ecuador. Pol. Con., 7(8), 2484-2499.
[12] Rodríguez, M. C. (2022). Expansión y hospederos de la Hemiparásita, Phoradendron nervosum, en el Campus de la Universidad ESPE, Quito, Ecuador. Revista Ciencia., 24(2), 17-30. doi: https://doi.org/10.24133/ciencia.v24i2.2699
[13] MECN. (2009). Ecosistemas del Distrito Metropolitano de Quito (DMQ). Serie de Publicaciones del Museo Ecuatoriano de Ciencias Naturales (MECN) - Fondo Ambiental del MDMQ, 6, 1-51.
[14] Duperier, E., Vallejo, R. y Yánez, G. (1995). Quito: Población y urbanización metropolitana 1982–2020. Dirección general de planificación.
[15] Jhu, M. y Sinha, N. (2022). Parasitic Plants: An Overview of Mechanisms by Which Plants Perceive and Respond to Parasites. Annual Review of Plant Biology, 73. doi: https://doi.org/10.1146/annurev-arplant-102820-100635
[16] Wong, S. Y., y Joling, J. (2021). Checklist of aroids (Alismatales, Araceae) from Sabah (Malaysian Borneo). Check List, 17(3), 931-974. doi: https://doi.org/10.15560/17.3.931
[17] Missouri Botanical Garden. (2015). Trópicos. http://www.tropicos.org
[18] R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/
[19] Sierra, R. (1999). Propuesta Preliminar de un Sistema de Clasificación de Vegetación para el Ecuador Continental. INEFAN/ GEF–BIRF y EcoCiencia.
Author notes

