Bioaccessibility as a tool for planning bioremediation of petroleum-polluted soil
La bioaccesibilidad como herramienta de planificación de la biorremediación de suelos contaminados con petróleo
ACI Avances en Ciencias e Ingenierías
Universidad San Francisco de Quito, Ecuador
Received: 14 May 2022
Accepted: 25 November 2022
Abstract: Soil contamination caused by petroleum-related oil spills is a relevant problem in the Ecuadorian Amazon region. Bioremediation is a usual technique to clean up the soil. In this study, treatment efficiency in soils contaminated by Total Petroleum Hydrocarbons (TPH) was evaluated using microcosms (natural attenuation vs. biostimulation) and field assays (landfarming and windrows with and without aeration). Moreover, the final TPH of each experiment was contrasted with the concentration of non-bioaccessible hydrocarbons, following the hypothesis that only the bio-accessible fraction of contaminant can be metabolized. The TPH remotion in field assays was higher than in microcosms assays, observing that aeration is a crucial factor. In microcosms, there were no statical differences between natural attenuation and biostimulation. There was no observed clear relation between non-accessible hydrocarbons and the end-point remediation. However, the method must be standardized according to the potential application of this parameter to set up feasible bioremediation goals.
Keywords: Bioremediation efficiency, petroleum-hydrocarbons, soil contamination, risk assessment, Ecuadorian Amazon region, oil liability.
Resumen: La contaminación de suelos por hidrocarburos de petróleo en la Amazonía ecuatoriana es un problema ambiental relevante. La biorremediación es ampliamente utilizada para remediar este suelo. En este estudio se evaluó la eficiencia de tratamiento al aplicar biorremediación en microcosmos (atenuación natural vs bioestimulación), y condiciones de campo (“landfarming” y biopilas con y sin aireación, cada uno) en suelos contaminados, con hidrocarburos totales de petróleo (TPH). Las concentraciones no removidas de TPH fueron comparadas con la concentración de hidrocarburos no bioaccesibles, cuantificados para cada tratamiento utilizando tres métodos de extracción no exhaustiva con alcoholes (1-propanol, 50% 1-propanol y 1-butanol), con la hipótesis de que solo las fracciones bioaccesibles pueden ser biodegradadas. Se observó una mayor eficiencia de remoción de TPH en los ensayos de campo que en los de laboratorio, determinándose que la aireación es un factor clave en la biorremediación. En los ensayos de laboratorio no se observó un efecto significativo al aplicar bioestimulación. No se observó una correlación entre los valores de bioaccesibilidad de hidrocarburos y los concentración final de TPH. Estos resultados sugieren que la bioaccesibilidad debería ser usada como herramienta para establecer metas de biodegradación factibles por lo que el método requiere ser estandarizado.
Palabras clave: Eficiencia de biorremediación, hidrocarburos de petróleo, suelo contaminado, análisis de riesgo, región amazónica ecuatoriana, pasivo ambiental.
INTRODUCTION
Modern society bases its economy on petroleum (and its derivatives); therefore, this natural resource is a crucial commodity in the financial system and global economy. Petroleum exploitation involves activities implying many potential environmental impacts [1], mainly affecting soil and water. Oil spills are difficult to avoid during petroleum extraction, processing, and delivery, even when all regulatory requirements are fulfilled [2]. Soil is a receptor of pollutants of anthropogenic origin that contribute to land degradation with negative consequences on the food chain, public health, and water and air quality [3,4]. Soil protection is key to achieving the Sustainable Development Goals proposed by the United Nations [5].
Bioremediation is a cost-effective and commonly used technique for treating soils and sediments contaminated with petroleum hydrocarbons [6]. However, the efficiency of this process is usually affected by many factors, such as microbial composition, the contaminant (toxicity, structure, concentration), environmental conditions (pH, salinity, nutrients availability), cometabolism, type of process (aerobic vs. anaerobic), mass transfer limitation, and bioavailability of contaminant [7].
Biodegradability is the assimilation of a compound (pollutant) by organisms for the biosynthesis of cellular components or complete mineralization to obtain energy or inorganic nutrients. The composition of the microbial community during the degradation process is dynamic, the predominant group being bacteria [8,9].
Favorable environmental conditions for the growth of microorganisms are necessary for bioremediation. Microorganisms need the proper nutrients to produce the necessary enzymes to break down the contaminants. Also, nutrients, pH, temperature, and moisture influence microbial growth. Therefore, the proper environmental conditions must be established (and maintained) to ensure that microorganisms can degrade a pollutant [1].
Recalcitrance and persistence are closely related. According to the European Environmental Agency, the term recalcitrant is “applied to pollutants which are not biodegradable or are only biodegradable with difficulty” [10]. Persistence is a broader concept; a chemical is persistent if it is not degraded by biological, chemical, or physical processes. Persistence, often assessed with bioaccumulation and toxicity, is a significantcomplex criterion for remediation assessment [11].
Bioavailability is the degree to which chemicals present in the soil may be taken up or metabolized by human or ecological receptors or are available for interactions with biological systems [12]. According to [13], bioavailability has two complementary sides: accessibility and chemical activity. Bioaccessibility describes the total fraction of contaminants potentially available over time [14]; this fraction reduces the time and cost associated with determining bioremediation treatment of contaminated soils [15].
On the other hand, chemical activity is measured with equilibrium sampling devices and quantifies the potential for spontaneous physicochemical processes (such as diffusion, sorption, or partitioning).
The assessment of these two aspects allows risk evaluation of polluted places and tailoring to specific protection goals. The main reason is that the mere presence of a contaminant, by definition, does not mean an actual risk or a measurable effect on an ecosystem [13,16].
At a field scale, bioremediation is performed either in situ or ex situ. In situ techniques have the remarkable advantage that the material is treated in the same place where the spill occurred; therefore, the transport of polluted soil (and associated costs) is avoided. On the other hand, ex situ techniques require the movement of polluted soils. Ex situ techniques are more efficient than others and, generally, require less time to complete the process. Moreover, their monitoring is easier, and so is ensuring proper conditions (among them, maintaining the homogeneity of the material) to facilitate the remediation process [17,18].
In the Ecuadorian Amazon region, around 2,550 pollution sources, categorized as tailing ponds, waste pits, and spills, were attributed to former oil industry activities in the country [19]. The estimated amount of polluted soil is around 5,317,808 m3. Up to July 2022, EP PETROECUADOR and its Project Amazonia Viva (P.A.V.) had eliminated 1,095 pollution sources and cleaned up to 1,431,000 m3 of soil. The average cost for each cubic meter of cleaned soil is 65.84 USD. Remediation results comply with Ecuadorian standards, which are presented in Table 1 [20]. P.A.V. had been using either landfarming or windrows for the remediation of soils since these strategies showed cost and social advantages.
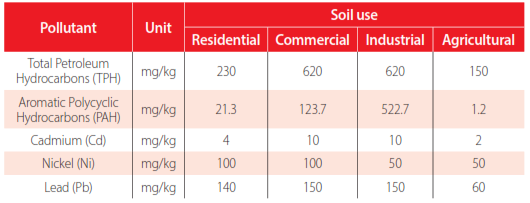
Standards for soil quality according to Ecuadorian legislation applicable to remediation of soil contaminated with petroleum-hydrocarbons. (Ministerio del Ambiente del Ecuador, 2015)
This study aimed to evaluate landfarming and windrows as bioremediation techniques for removing Total Petroleum Hydrocarbons (TPH) from soils under field conditions in the Ecuadorian Amazon rainforest. These results were compared with TPH removals achieved under laboratory conditions. At this stage, bioaccessibility estimations were proposed as a theoretical maximum of TPH removal to calculate efficiencies. The importance of bioavailability in setting up feasible bioremediation goals is discussed.
MATERIALS AND METHODS
Characteristics of polluted soil and treatment center
A mixture of 1,000 m3 of contaminated soil was obtained from a pollution source of petroleum activities located in the province of Francisco de Orellana, in the Ecuadorian Amazon region. The soil was taken to the Treatment Center CGP_YU_04 (E 301908.7556 W 9946516.921) for the bioremediation assays. CGP_YU_04 consisted of a flat surface with a slope of 2%, was waterproofed with compacted clay, and had a perimeter canal for retrieving and treating leach. Relevant meteorological data during the trials were: average semi-annual precipitation of 1,774.1 mm and semi-annual average absolute maximum temperature of 34.3 °C.
The soil had an average density of 1.35 g/cm3 and a pH of 5.67. According to the USDA texture triangle, the soil is classified as clay-loam. Previous to the assays, the soil was homogenized for three days using a hydraulic backhoe loader (CATERPILLAR model 420- E), and stones and any other debris were removed.
Assays under laboratory conditions
One kilogram of soil was collected from the stock located at CGP_YU_04, keeping sterile conditions. Prior to the experiment, it was passed through a 2-mm stainless steel sieve. Biodegradation assays using slurry-phase microcosms were done following the methodology proposed by Kuppusamy et al., 2016 [17].
Two treatments were carried out using 1 L sterile glass bottles: Biostimulation (TL0) with 100 cm3 of soil, 200 cm3 of distilled water, and 15 cm3 of basal mineral medium without carbon, supplemented with crude oil of “Planta de Tratamiento de crudo” (Mills et al.,1978)[21]; and No-biostimulation (TL1), without the addition of medium. Microcosm experiments were incubated at 30 °C in the dark and stirred at 120 rpm using two Wise Cube shaking incubators model WIS-20R.
To accommodate the measurement of many parameters throughout the experiment, multiple replicate microcosms were set up for each treatment and sacrificed after the sampling.
In total, 36 microcosms were made (2 treatments x 3 replicates x 9 samplings). Sampling was performed on days 1, 7, 14, 21, 28, 56, 72, 84, and 112. Assays were done at Centro de Investigación de Tecnologías Ambientales in Joya de Los Sachas, Orellana Province (Ecuador).
Assays under field conditions
The windrows and landfarming techniques, with and without mechanical aeration, were tested, for comparative purposes, in experimental units of 120 m3. The following were the tested treatments: Windrows without aeration (W), Windrows with aeration (W+A), Landfarming without aeration (L), and Landfarming with aeration (L+A) (Fig. 1). The treatments were tested in triplicate. Volumes were determined through R.T.K. surveying technology using a Trimble G.P.S. model TSC3. A windrow unit had a length of 20 m, a width of 2.5 m, and a height of 2.4 m. A landfarming unit had a length of 40 m, a width of 7.5 m, and a height of 0.4 m.

Figure 1.
Bioremediation field assays. A) Windows. B) Landfarming. Source: Authors.
For landfarming, aeration was planned biweekly and carried out by plowing with a MASSEY-FERGUSON tractor model 4291 and the same backhoe described before for windrows. Static aeration was not possible for windrows since the soil is easily compacted in the absence of movement due to its clay content, the influence of rainwater, and high temperatures.
The sampling of each experimental unit was done according to the guidelines detailed in [22]. After the aeration, one kilogram of soil composed of 10 subsamples was collected for each treatment. For the windrows, subsamples were collected following a zig-zag pattern from the base, center, and top. For landfarming, samples were collected from the surface layer (20 cm in depth) following an X pattern.
Samples were collected on days 1, 7, 14, 28, 56, 84, 112, and 140. Due to adverse climatic conditions (heavy rain) and unavailability of machinery for aeration, no samples were collected on days 21 and 72.
Analytical methods
Before chemical and microbiological analysis, the soil samples were air-dried at room temperature. For each experiment (laboratory and field conditions), the following parameters were analyzed: TPH, Hydrocarbon-Degrading Microorganisms (HDM), andTotal- Heterotrophic Microorganisms (THM). Non-Bioaccesible-Hydrocarbons concentration was quantified only at the beginning (day 7) because this parameter provides a theoretical approach to the amount of contaminant potentially available over time [15]. The pH value was measured at the beginning and end using the electrometry method EPA 9045D [23].
Three grams of soil were used to determine TPH concentration following the method EPA 8440 [24] with a Nicolet™ iS™ 5 FTIR Spectrometer (Thermo Scientific™). To quantify Non-Bioaccesible-Hydrocarbons, the procedure described by [15] was applied. This procedure is based on non-exhaustive extractions with different alcohols. In short, 5 g of soil was mixed with 50 mL of alcohol at 150 rpm for 60 min (temperature 25 °C). The following alcohols were tested: 1-butanol (99.5%, Fischer Scientific), 1-propanol (99.5%, Sigma-Aldrich), and 1-propanol (50%, Sigma-Aldrich). Afterward, the suspension was centrifuged at 3,000 G for 5 min. Non-bioaccessible hydrocarbons were quantified in the pellet using the Method EPA 8440 [24]. The content of Bioaccesible-Hydrocarbons was obtained by the arithmetical subtraction of Non-Bioavailable Hydrocarbons from TPH.
HDM was determined by counting colonies growing on a Solid Basal Mineral Medium (NH4Cl: 2.0 g/L, KH2PO4: 0.89 g/L, Na2HPO4: 1.25 g/L, FeCl3: 0.6 mg/L), as described in [25], and supplemented with petroleum as the only carbon source (23° API coming from the deposit tanks of EP PETROECUADOR Sacha-oil facilities, province of Orellana, Ecuador). Plates were incubated for up to 48 h at 30 °C, and THM was determined by counting colonies growing on Nutrient Agar (Merck) [12].
Statical analysis
As mentioned before, all analyses were carried out in triplicate. Using the individual analysis results, the average, standard deviation, and percentage error were calculated for each parameter. By the use of the Infostat software, degradation curves of each treatment were compared to each other by applying ANOVA and a Tukey test with a significance of 0.05.
RESULTS
[15] mentioned that THM prefers a nearly neutral pH; they observed the maximum degradation of petroleum hydrocarbons at a pH of 7.5.
At the laboratory scale, the initial pH value of the soil was 5.67 and was easily adjusted to pH 7.00 by adding a 0.1 N NaOH solution. A periodical adjustment was necessary. The final pH values were between 6.78 and 6.91. At the field scale, the initial pH values of soils were between 4.64 and 5.57, and they reached values of 5.02 to 5.60 with the addition of agricultural lime. In this case, a pH value of 7.00 was not achieved.
TPH remotion
The degradation curve of TPH in laboratory assays shows a total TPH-remotion of 25.15% in TL0 and 26.50% in TL1. Both curves have a correlation index of 0.877, without a statical difference (P<0.05). A fluctuating TPH was observed with periodic increments (days 7, 21, and 72) and decrements (days 14, 56, and 112). No stabilization of the curve was observed. Day 112 was considered the end of the experiment in concordance withobservations in real scenarios in the field (Fig. 2A).
On the other hand, field assays show TPH-remotion of 11.2, 27.9, 28.6, and 43.6% for W, W+A, L, and L+A, respectively. Three different statistical groups were found—W+AIR and L+AIR, W, and L—evidence that aeration during soil treatments is a crucial factor in TPH remotion (see Fig. 2B). Similarly to laboratory assays, fluctuating TPH was observed, with two increments on days 28 and 112. TPH at day 140 was considered the end-point, with TPH comparable today 84. Tables S1 and S2 of Supplementary Material show the rates of TPH-remotion in laboratory and field assays.
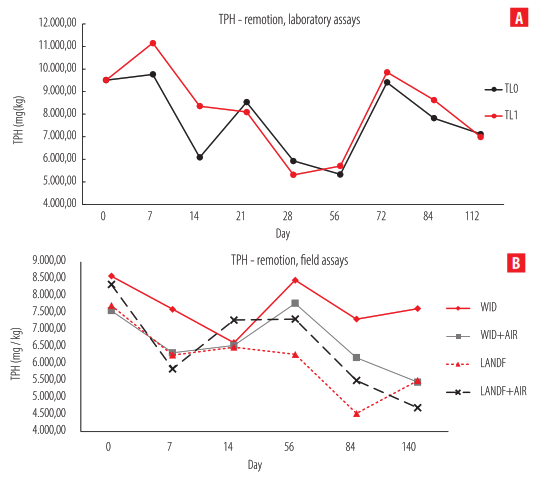
Figure 2.
TPH-remotion curves. A) Laboratory assays. B) Field assays.
Prediction of end-point remediation using non-bioaccessibility of hydrocarbons
A correlation was expected between the non-accessible hydrocarbon concentration and the final TPH (end-point remediation). However, the three alcohol-extraction methods used to quantify the non-bioaccessibility hydrocarbons do not show a clear correlation to the final TPH, with correlation coefficients of 0.23, 0.45, and 0.73 when the extraction was performed with 1-butanol, 1-propanol, and 50% 1-propanol, respectively (Table 2).
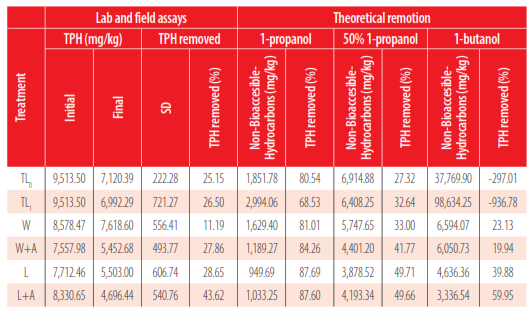
Non-bioaccessible Hydrocarbons quantified using three alcohol-extraction protocols.
The non-bioaccessible hydrocarbons detected using 50% 1-propanol were comparable to final TPH in treatments TL0, TL1, and L+A, while the results with the 1-butanol extraction were related to W, W+A, and L. The non-bioaccessible hydrocarbon method determined with 1-propanol did not relate to any treatment (Fig. 3).
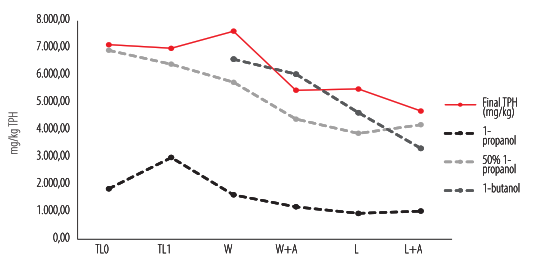
Figure 3.
Prediction of end-point remediation using 3-alcohol extraction methods of non-accessible hydrocarbons. Data of 1-butanol on treatments TL0 and TL1 are not shown because values are higher than the initial TPH.
Growth curves of microorganisms
For laboratory experiments, growth curves for THM and HDM in the treatment TL0 and TL1 did not show statistical differences (p=0.517 and p= 0.346, respectively). The population of THM varies from 6.15x104 to 1.03x106 CFU/g, and HDM varies from 1.6x104 to 1.05x107 CFU/g.
Tables 3 and 4 show a low correlation coefficient between the TPH of each treatment and the logarithm of count CFU of THM and HDM, obtaining correlation coefficients less than 0.15.
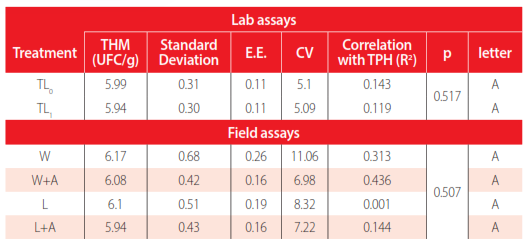
ANOVA Friedman (0.05) of THM in lab and field assays.
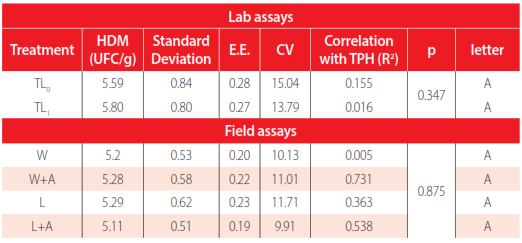
ANOVA Friedman (0.05) of HDM in lab and field assays.
In the same way, in the field assays, four treatments had similar results without a statistical difference, p= 0.5071 for THM and p=0.8751 for HDM. A higher correlation coefficient was obtained in count CFU of HDM to windrows with air with TPH (R2= 0.731). In the remaining treatments, the correlation coefficient was less than 0.44.
DISCUSSION
Evaluation of landfarming and windrows as bioremediation techniques
In this study, bioremediation in microcosms reveals that biostimulation does not produce a significantly better performance than natural attenuation. In contrast to other research [26,27, 28], where it is observed that bioremediation in laboratory conditions gets a similar or greater remotion of TPH compared to field assays, in this study, the percentages of TPH remotion in microcosms were lower than field assays except for windrows treatment. This result suggests that environmental conditions typical of the Amazon region, specifically the sun radiation, high humidity, and high temperatures that raise soil temperature, could promote biodegradation in field assays. Previously it was observed that both physicochemical (temperature, initial hydrocarbon concentration) and biological variables play a fundamental role in TPH remotion [29].
Treatment efficiency under field conditions varies according to the treatment method: landfarming or windrows. Overall, landfarming had a better performance than windrows for TPH removal: 43.62% of efficiency vs. 27.86% for the respective aerated treatments. Even the non-aerated landfarming treatment (28.65% of efficiency) obtained similar results as the aerated windrows. This fact implies that landfarming is a feasible alternative to treatment; it is impossible to give enough aeration. However, the main disadvantage is the necessary area. In our trials, we needed 300 m2 of surface to treat 120 m3 of soil for landfarming, which means 2.5 m2/m3 of soil treated.
Meanwhile, for windrows, the same volume was treated in 50 m2, equivalent to 0.42 m2/m3 soil treated. Therefore, landfarming needs six times more space than windrows. Accordingly, it could be improved by increasing the height of the landfarming unit. Therefore, having an appropriate machine to work deep enough and remove the complete volume of soil in treatment is necessary.
Comparing the non-aerated vs. aerated treatments for each case (W vs. W+A and L vs. L+A) shows that air injection is essential for effective biodegradation. However, other research has reported that a 10–40% oxygen level is required for effective hydrocarbon biodegradation [30].
In contrast with other studies of bioremediation counting of THM and HDM, microbial countings do not correlate with the TPH degradation rates [31]. No significant difference was found between the treatments in the field and laboratory assays. However, the population of THM and HDM had moments of increase throughout the 112 days of the project, which indicates that the number of microbial populations was not influenced by the bioremediation treatments, suggesting that the composition of the microbial population is affected by the environmental conditions and the composition of the hydrocarbons. Similar results have been reported in [32].
Prediction of end-point remediation using non-bioaccessibility of hydrocarbons
The 50% 1-propanol extraction method showed a high correlation coefficient with the final TPH in this study. However, this value is not near zero to accurately be used to predict biodegradation end-points, as suggested by [33].
In previous research about the application of non-bioaccessible hydrocarbons to predict end-point bioremediation, fractions of hydrocarbons were analyzed separately, obtaining specific correlation coefficients for each one [15,34,35]. This study aimed to quantify the bioaccessibility of TPH related to estimating the maximum TPH removal in field assays. The results obtained reflect the analysis for a few samples (three for each treatment); therefore,the next step is to standardize the extraction method using more samples and different soils. [36] obtained similar results by analyzing Polycyclic Aromatic Hydrocarbons.
This study was performed in 2018 with the available information [15]; however, the international standard ISO 16751:2020 (2020) [37] for assessing the potentially bioavailable fraction of non-polar organic compounds could be used.
The local environmental legislation determines remediation goals, and in cases like Ecuador, this is related to the removal of a pollutant and measured for its total concentration. Therefore, and according to Table 2, if the value of the bioavailable fraction is not enough to fulfill the requirement of the environmental legislation, a non- biological treatment is needed to achieve the remediation goals, either as the primary treatment or as a complementary one. The latter drives the necessity to incorporate Bioavailability analysis before starting remediation treatments. A decision matrix is presented in Figure 4 for the decision of the chosen technology implemented for the remediation process.
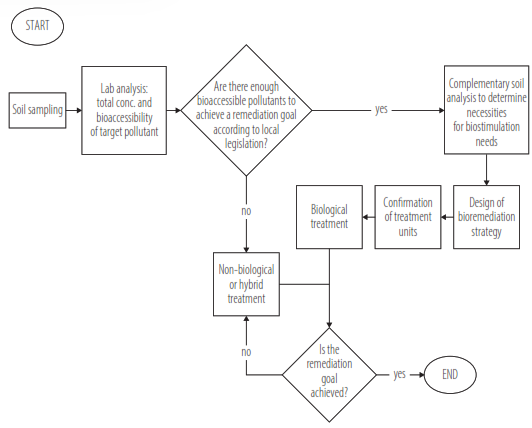
Figure 4.
Decision matrix for choosing technology in the soil remediation process. Source: Authors.
The Ecuadorian environmental legislation determines that remediation goals are related to the total concentration of contaminants. Therefore, if the bioavailable fraction is not enough to fulfill the requirement of the environmental legislation limits, a non- biological treatment is needed to achieve the remediation goals. So, it will be necessary to incorporate bioavailability before starting remediation treatments to estimate the treatment time and the other resources needed.
Scientific developments in bioavailability are not always translated into approaches ready to use for regulators; thus, within the regulatory frameworks are uncharted fields regarding regulation and approval of organic chemicals.
A survey conducted in the UK about bioavailability applicability in risk-based regulation contacted 375 local authorities. 78% of the respondents expressed concern that the lack of statutory guidelines was hampering the application of bioavailability to the risk assessment and management of contaminated land. However, bioavailability in regulatory frameworks is still a fair limit in some countries (the Netherlands and Australia) [11,14, 38].
In this research, neither Landfarming nor Windrows reached TPH concentrations to meet Ecuadorian standards, being necessary to complement biological processes with other technologies to meet those legal requirements. For example, a Life Cycle Assessment performed on another remediation project in a nearby area (Joya de Los Sachas, Ecuador) recommended lowering the use of vehicles and heavy machinery and making thoughtful route planning to maintain the sustainability of the whole process [39]. Then, the question remains on whether it is necessary to keep working on the pollutant degradation if the toxicity associated with bioaccessible hydrocarbons is not a problem since most bioaccessible fractions were already (bio)degraded. These additional works may negatively impact the sustainability of the project.
CONCLUSIONS
Landfarming was more efficient than windrows. In all cases, aeration is a decisive factor for TPH removal; however, it is necessary to make a previous characterization that includes bioaccessibility measurements to determine the biodegradable fractions and establish realistic remediation goals.
It is necessary to apply a standardization on the method to quantify the bioaccessibility of hydrocarbons in soils using the protocol described at ISO 16751. Moreover, further tailor-made research on field conditions is needed to corroborate what the literature says: bioavailability, biodegradability, and toxicity are highly correlated. Developing this research may lead to obtaining analytical tools to prioritize the treatment of pollutants with high risk in contrast to the ones with higher concentrations.
Bioaccessibility and bioavailability are concepts that should be integrated into the Ecuadorian environmental legislation to improve the assessment of remediation projects.
ACKNOWLEDGMENTS
To EP PETROECUADOR for funding this project. To Marivi Vargas, Geovanny Ureña, Luis Quesada, Luis Solórzano, Edison Calle, and Patricio Gómez Ortega for their help with laboratory and field trials. The authors acknowledge the support of coworkers from PAV- Auca and PAV-SIMP for their help with logistics to carry out field activities.
AUTHOR CONTRIBUTIONS
Conceptualization, D.H.L.; methodology, D.H.L.; investigation, D.H.L., J.L.M., P.Y.T., D.M.T., K.G.V.; data curation, D.H.L., K.G.V., P.V.J.; writing—original draft preparation, D.H.L.; writing—review and editing: K.G.V., J.U.U., P.V.J.; project administration, D.H.L., J.C.L.M.; funding acquisition, K.G.V. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
[1] Vidali, M. (2001). Bioremediation. An overview. Pure and Applied Chemistry, 73(7), 1163–1172. https://doi.org/10.1351/pac200173071163
[2] Fuentes, S., Méndez, V., Aguila, P., & Seeger, M. (2014). Bioremediation of petroleum hydrocarbons: Catabolic genes, microbial communities, and applications. Appl. Microbiol. Biotechnol., 98, 4781–4794. https://doi.org/10.1007/s00253-014-5684-9
[3] Nunes, D., Salgado, A., Gama-Rodrigues, E., Taketani, R., Cunha, C., & Da Servulo, E. (2020). Use of plant materials for the bioremediation of soil from an industrial site. Part A. J. Environ. Sci. Health, 55, 650–660. https://doi.org/10.1080/10934529.2020.1726695
[4] Prăvălie, R. (2021). Exploring the multiple land degradation pathways across the planet. Earth-Sci. Rev., p. 220, 103689. https://doi.org/10.1016/j.earscirev.2021.103689
[5] Paleari, S. (2017). Is the European Union protecting soil? A critical analysis of Community environmental policy and law. Land Use Policy, 64(3), 163–17. https://doi.org/10.1016/j.landusepol.2017.02.007
[6] Ajona, M., & Vasanthi, P. (2021). Bioremediation of petroleum contaminated soils – A review. Materials Today: Proceedings, 45(1), https://doi.org/10.1016/j.matpr.2021.01.949
[7] Kebede, G., Tafese, T., Abda, E., Kamaraj, M., & Assefa, F. (2021). Factors Influencing the Bacterial Bioremediation of Hydrocarbon Contaminants in the Soil: Mechanisms and Impacts. (Y. He, Ed.) Journal of Chemistry (9823362), 17 pages. https://doi.org/10.1155/2021/9823362
[8] Cui, J., He, Q., Liu, M., Chen, H., Sun, M., & Wen, J. (2020). Comparative Study on Different Remediation Strategies Applied in Petroleum-Contaminated Soils. Int. J. Environ. Res. Public. Health, 17, 1606. https://doi.org/10.3390/ijerph17051606
[9] Shahi, A., Aydin, S., Ince, B., & Ince, O. (2016). Evaluation of microbial population and functional genes during the bioremediation of petroleum-contaminated soil as an effective monitoring approach. Ecotoxicology and Environmental Safety, 125, 153-160. https://doi.org/10.1016/j.ecoenv.2015.11.029
[10] European Environment Agency. (2021). Glossary. Retrieved from https://www.eea.europa.eu/help/glossary/chm-biodiversity/recalcitrant
[11] Ortega-Calvo, J., Stibany, F., Semple, K., Schaeffer, A., Parsons, J., & Smith, K. (2020). Why Biodegradable Chemicals Persist in the Environment? A Look at Bioavailability. In J. Ortega-Calvo, J. Parsons, & J. Robert, Bioavailability of Organic Chemical in Soil and Sediment. The Handbook of Environmental Chemistry. (p. 243-265). Springer International Publishing. https://doi.org/10.1007/698_2020_586
[12] ISO 17042:2008. (2008). Soil quality - Requirements and guidance for the selection and application of methods for the assessment of bioavailability of contaminants in soil and soil materials.
[13] Reichenberg, F., & Mayer, P. (2006). Two complementary sides of bioavailability: Accessibility and chemical activity of organic contaminants in sediments and soils. Environmental Toxicology and Chemistry, 5, 1239-1245. https://doi.org/10.1897/05-458r.1
[14] Ortega-Calvo, J., Harmsen, J., Parsons, J., Semple, K., Aitken, M., Ajao, C., Eadsforth, C., Galay-Burgos, M., Naidu, R., Oliver, R., Peijnenburg,W., Römbke, J., Streck, G., &Versonnen, B. (2015). From Bioavailability Science to Regulation of Organic Chemicals. Environmental Science & Technology, 49, 10225-10264. http://dx.doi.org/10.1021/acs.est.5b02412
[15] Dandie, C., Weber, J., Aleer, S., Adetutu, E., Ball, A., & Juhasz, A. (2010). Assessment of five bioaccessibility assays for predicting the efficacy of petroleum hydrocarbon biodegradation in aged contaminated soils. (Elsevier, Ed.) 81, 1061-1068. http://dx.doi.org/10.1016/j.chemosphere.2010.09.059
[16] Peijnenburg, W. (2020). Implementation of Bioavailability in Prospective and Retrospective Risk Assessment of Chemicals in Soils and Sediments. In J. Ortega-Calvo, Bioavailability of Organic Chemicals in Soil and Sediment, The Handbook of Environmental Chemistry. (p. 391-442). Springer International Publishing. https://doi.org/10.1007/698_2020_516
[17] Kuppusamy, S., Thavamani, P., Megharaj, M., & Naidu, R. (2016). Bioaugmentation with Novel Microbial Formula vs. Natural Attenuation of a Long-Term Mixed Contaminated Soil—Treatability Studies in Solid- and Slurry-Phase Microcosms. Water Air Soil Pollution, 227:25. http://dx.doi.org/10.1007/s11270-015-2709-7
[18] Tomei, M., & Daugulis, A. (2013). Ex Situ Bioremediation of Contaminated Soils: An Overview of Conventional and Innovative Technologies. Crit. Rev. Environ. Sci. Technol., 43, 2107–2139. https://doi.org/10.1080/10643389.2012.672056
[19] PRAS, P. d. (20 de Julio de 2022). Ministerio del Ambiente y Transición Ecológica del Ecuador. Obtenido de http://pras. ambiente.gob.ec/documents/228536/737569/Plan+de+Reparaci%C3%B3n+de+Pacayacu+-+Final-1+_1_ Ministerio del Ambiente del Ecuador. (2015). Acuerdo Ministerial 097. Anexos del Texto Unificado de Legislación Secundaria del Ministerio del Ambiente.
[20] Mills, A., Breuil, C., & Colwell, R. (1978). Enumeration of petroleum-degrading marine and estuarine microorganisms by the most probable number method. Can J Microbiol, pp. 24, 552–557.
[21] ISO 10381-6:2009. (2014). Calidad del Suelo, Muestreo. Parte 6:guía para el muestreo, la manipulación y la conservación de suelos en condiciones aerobias, para la evaluación en el laboratorio de procesos, biomasa y diversidad microbiana.
[22] Environmental Protection Agency. (1996b.). Soil and Waste pH. Method 9045D.
[23] Environmental Protection Agency. (1996a). Total Recoverable Petroleum Hydrocarbons By Infrared Spectrophotometry. Method 8440.
[24] Delille, D., & Coulon, F. (2008). Comparative Mesocosm Study of Biostimulation Efficiency in Two Different Oil- Amended Sub-Antarctic. Soils. Microb. Ecol., 56, 243–252. https://doi.org/10.1007/s00248-007-9341-z
[25] Diplock, E., Mardin, D., Killham, K., & Paton, G. (2009). Predicting bioremediation of hydrocarbons: Laboratory to field scale. Environ. Pollut., 157, 1831–1840. https://doi.org/10.1016/j.envpol.2009.01.022
[26] Suja, F., Rahim, F., Taha, M., Hambali, N., Razali, M., Khalid, A., & Hamzah, A. (2014). Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. International Biodeterioration & Biodegradation, 90, 115-122. https://doi.org/10.1016/j.ibiod.2014.03.006
[27] Szulc, A., Ambrozewicz, D., Sydow, M., Lawniczak, L., Piotrowska-Cyplik, A., Marecik, R., & Chrzanowski, L. (2014). The influence of bioaugmentation and biosurfactant addition on bioremediation efficiency of diesel-oil contaminated soil: Feasibility during field studies. Journal of Environmental Management, 132, 121-128. http://dx.doi.org/10.1016/j.jenvman.2013.11.006
[28] Martínez Álvarez, L. M., Ruberto, L., Gurevich, J., & Mac Cormack, W. (2018). Environmental factors affecting reproducibility of bioremediation field assays in Antarctica. Cold Regions Science and Technology. https://doi.org/10.1016/j.coldregions.2019.102915
[29] Clarkson, M. (2015). Bioremediation and Biodegradation of Hydrocarbon Contaminated Soils: A Review. I.O.S.R. Journal of Environmental Science, Toxicology and Food Technology, 9(11), 38–45.
[30] Wu, M., Wei, L., Dick, W. A., Ye, X., Chen, K., Kost, D., & Chen, L. (2017). Bioremediation of hydrocarbon degradation in a petroleum-contaminate soil and microbial population and activity determination. Chemosphere, 169, 124-130. http://dx.doi.org/10.1016/j.chemosphere.2016.11.059
[31] Bento, F., Camargo, F., Okeke, B., & Frankenberger, W. (2005). Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation, and bioaugmentation. Bioresource Technology, 96(9), 1049- 1055. https://doi.org/10.1016/j.biortech.2004.09.008
[32] Stroud, J. L., Paton, G. I., & Semple, K. T. (2008). Linking chemical extraction to microbial degradation of 14C-hexadecane in soil, Environmental Pollution, 156, 474–481. https://doi.org/10.1016/j.envpol.2008.01.018
[33] Stroud, J. L., Paton, G. I., & Semple, K.T. (2009). Predicting the biodegradation of target hydrocarbons in the presence of mixed contaminants in soil. Chemosphere, pp. 74, 563–567. https://doi.org/10.1016/j.chemosphere.2008.09.071
[34] Juhasz, A., Aleer, S., & Adetutu, E. (2014). Predicting PAH bioremediation efficacy using bioaccessibility assessment tools: Validation of PAH biodegradation e bioaccessibility correlations. International Biodeterioration & Biodegradation, 95, 320-329. https://doi.org/10.1016/j.ibiod.2014.09.003
[35] Rostami, I., & Juhasz, A. (2013). Bioaccessibility-based predictions for estimating PAH biodegradation efficacy-Comparison of model predictions and measured end-points. International Biodeterioration & Biodegradation, 85,323-330.
[36] ISO 16751:2020 (2020). Soil quality - Environmental availability of non-polar organic compounds- Determination of the potentially bioavailable fraction and the non-bioavailable fraction using a strong adsorbent of complexing agent.
[37] Latawiec, A., Swindell, A., Simmons, P., & Reid, B. (2011). Bringing Bioavailability into Contaminated Land Decision Making: The Way Forward?. Critical Reviews in Environmental Science & Technology, 41, 52–77. http://dx.doi.org/10.1080/00102200802641780
[38] Garcia-Villacis, K., Ramos-Guerrero, L., Canga, J. L., Hidalgo-Lasso, D., & Vargas-Jentzsch, P. (2021). Environmental Impact Assessment of Remediation Strategy in an Oil Spill in the Ecuadorian Amazon Region. Pollutants, 1(4), 234- 252. https://doi.org/10.3390/pollutants1040019