Sección B: Ciencias Biológicas y Ambientales
The role of aerobic respiration in the life cycle of Escherichia coli: Public health implications
El rol de la respiración aeróbica en el ciclo de vida de Escherichia coli: Implicaciones para la salud pública
ACI Avances en Ciencias e Ingenierías
Universidad San Francisco de Quito, Ecuador
ISSN: 1390-5384
ISSN-e: 2528-7788
Periodicity: Bianual
vol. 7, no. 2, 2014
Received: 02 June 2015
Accepted: 05 October 2015
Corresponding author: gtrueba@usfq.edu.ec

This work is licensed under Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International.
Abstract: The anaerobic intestinal setting of warm-blooded animals is considered E. coli’s main habi-tat. However E. coli transmission to new hosts requires the release of bacterial cells to an aerobic environment; we postulate that E. coli uses aerobic respiration to multiply in fecal matter during this phase of the cycle. To test this idea, we incubated fresh chicken fecal matter in aerobic and anaerobic settings and showed that E. coli counts increased signifi-cantly when fecal matter was incubated in the presence of oxygen. Our results suggest that aerobic growth in fecal matter outside of the host may be a crucial phase of E. coli’s natural cycle. This feature may extend to pathogenic members of the Enterobacteriaceae family.
Keywords: Escherichia coli, aerobic respiration, fecal matter, environment.
Resumen: Se piensa que el hábitat principal de E. coli, es anaerobio (los contenidos intestinales de animales de sangre caliente). Sin embargo la transmisión de E. coli de un hospedador a otro requiere la eliminación de esta bacteria (por medio de las heces) a un medio rico en oxígeno. Nosotros proponemos que E. coli usa la respiración aerobia para multiplicarse masivamente en las heces. Para probar esta hipótesis nosotros incubamos heces fecales de aves en presencia y ausencia de oxígeno. Nuestros resultados sugieren que el crecimiento aerobio en las heces es una parte crucial en el ciclo natural de esta bacteria. Esta caracterís- tica puede ser común a otros miembros de la familia Enterobacteriaseae.
Palabras clave: Escherichia coli, respiración aeróbica, heces fecales, ambiente.
Introduction
The anaerobic setting within the large intestine of warmblooded animals is considered the primary habitat of Escherichia coli; here E. coli populations readily replicate. However, a crucial part of E. coli’s life cycle is fecaloral transmission between animal hosts. Large numbers of E. coli cells are released to the environment (with fecal matter) and they reach the new host through water or food. Outside the hosts these bacteria are exposed to a largely aerobic setting.
Escherichia coli transmission to other hosts depends mainly on environmental conditions and its aptitude to survive in this aerobic environment [1], although some E. coli strains may have evolved to grow outside of the host [2]. At the same time most E. coli strains survive short periods of time outside the host [1], this is why E. coli is considered a good indicator of fecal contamination.
Escherichia coli, and other members of the family Enterobacteriaceae, use different metabolic pathways (anaerobic respiration, microaerobic respiration and fermentation) to obtain energy from organic compounds (in animal’s guts), under either low oxygen or anaerobic conditions [4–6]; therefore it is intriguing that aerobic respiration is a universal property in E. coli strains.
Although aerobic respiration is the most efficient way to obtain energy from organic matter [3, 4], it is energetically demanding as it requires many genes and the synthesis of several molecules that are part of complex systems such as: signal transduction, gene transcription and electron transport [1, 4, 6]. It is paradoxical that a bacterium which replicates mainly in a microaerophilic and anaerobic habitats, conserves genes for growth un- der fully aerobic conditions; bacterial genes are prone to inactivation unless there is a strong selective pressure
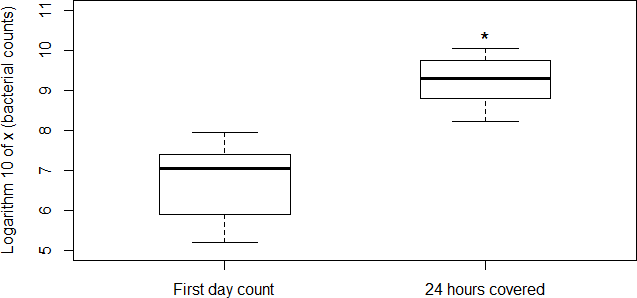
Figure 1
Boxplot using log 10 scale shows counts of Escherichia coli colonies per gram of fresh fecal matter First day and in fecal matter incubated in the bench for 24 hours at room temperature Asterisk indicates statistically significant difference as assessed by the ttest t619 pvalue4469e05
7
In the other hand, a previous report has demonstrated that E. coli can grow in fecal matter outside of the host and the authors suggested that aerobic respiration may be important component of this growth [8]. In this study we tested the hypothesis that E. coli uses oxygen to quickly multiply in fecal matter. We argue that using its resources for this quick amplification may enhance its chances of finding another host.
Materials and Methods
Fresh stool samples were obtained from a chicken [n=8]. Each sample was separated in two aliquots and weighed
separately. The first aliquot was immediately plated out and cultured for 24 hours at 37 ◦C in Chromocult Coliforms agar (Merck@); E coli was identified by β- glucoronidase activity. The second aliquot was placed into a Petri dish and left in a 21 ◦C after which this aliquot was cultured as previously.
In a subsequent experiment, fresh chicken stool samples were collected [n=10], separated in two aliquots and weighed. One part was placed in an open pouch and the other was placed in an anaerobic pouch (BD Gas- Pak EZ Anaerobe Pouch System, 260683). The samples were left for 24 hours in a 21◦C environment. E. coli were enumerated as in the previous experiment. Statistical analyses and figures were carried out in the RStudio, Inc.
Results y Discussion
Colony counts in fecal samples incubated for 24 hours at room temperature were significantly higher than colony counts of fresh fecal matter (Figure 1). To determine whether oxygen was involved in the high growth rates outside the intestine, a second experiment was carried out under both an aerobic and anaerobic conditions. The samples exposed to an aerobic environment showed a significant higher growth than those cultured under anaerobic conditions (Figure 2).
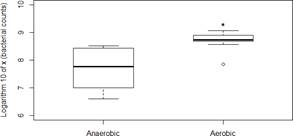
Figure 2
Boxplot using log 10 scale shows counts of Escherichia coli colonies per gram of feces after 24 hours of growth in anaerobic and aerobic conditions Asterisk indicates statistically significant difference as assessed by the ttest t401 pvalue 000154
Our experiments support the idea that the E. coli population multiply in fecal matter in the presence of oxy- gen, which may increase the chances of E. coli to colonize new hosts. The approximate increase in colonies (at least 10 fold) suggests that aerobic replication in fecal matter is a critical part in E. coli’s natural cycle (and possibly in the cycles of other members of Enterobacteriaceae including pathogens such as Salmonella).
Escherichia coli is a minor component of the animal microbiome probably because of high competition with anaerobic bacteria such as Bacterioides or Clostridium; however, once the intestinal content is exposed to oxy- gen, E. coli gains the competitive advantage and over- grows anaerobic bacteria. We propose that aerobic growth in fecal matter is a critical phase in E. coli’s natural life cycle. These findings underline the importance of a neglected phenomenon which has large implications in environmental sanitation and public health.
References
[1] Savageau, M. 1983. “Escherichia coli habitats, cell types, and molecular mechanisms of gene control”. American Naturalist, 122: 732-744.
[2] Ksoll, W.; Ishii, S.; Sadowsky, M.; Hicks, R. 2007. “Presence and sources of fecal coliform bacteria in epilithic periphyton communities of Lake Superior”. Applied and Environmental Microbiology, 73: 3771-3778.
[3] Hadjipetrou, L.; Stouthamer, A. 1965. “Energy pro-duction during nitrate respiration by Aerobacter aero-genes”. Journal of General Microbiology, 38: 29-34.
[4] Bueno, E.; Mesa, S.; Bedmar, E.; Richardson, D.; Del-gado, M. 2012. “Bacterial adaptation of respiration from oxic to microoxic and anoxic conditions: redox con-trol”. Antioxidants & Redox Signaling, 16: 819-852.
[5] Jones, S.; Gibson, T.; Maltby, R.; Chowdhury, F.; Stew-art, V.; Cohen, P.; Conway, T. 2011. “Anaerobic respi-ration of Escherichia coli in the mouse intestine”. Infec-tion and Immunity, 79: 4218-4226.
[6] Govantes, F.; Albrecht, J.; Gunsalus, R. 2000. “Oxy-gen regulation of the Escherichia coli cytochrome d ox-idase (cydAB) operon: roles of multiple promoters and the Fnr-1 and Fnr-2 binding sites”. Molecular Microbi-ology, 37: 1456-1469.
[7] Mira, A.; Ochman, H.; Moran, N. 2001. “Deletional bias and the evolution of bacterial genomes”. Trends in Genetics, 17: 589-596.
[8] Russell, J.; Jarvis, G. 2001. “Practical mechanisms for interrupting the oral-fecal lifecycle of Escherichia coli”. Journal of Molecular Microbiology and Biotechnology, 3: 265-272.
Author notes
gtrueba@usfq.edu.ec

