Natural products as a source of drugs on Ecuador's essential medicines list
Los productos naturales como fuente de fármacos en la lista de medicamentos esenciales de Ecuador
ACI Avances en Ciencias e Ingenierías
Universidad San Francisco de Quito, Ecuador
Received: 12 March 2020
Accepted: 10 December 2021
Resumen: En este trabajo se presenta el análisis del origen de 391 ingredientes activos de la 9na lista de medicinas esenciales de Ecuador, utilizando el sistema propuesto por Newman y Cragg con ciertas modificaciones con el propósito de destacar la contribución de los productos naturales en el descubrimiento y desarrollo de los fármacos utilizados en el sistema de salud pública ecuatoriana. Los ingredientes activos de origen natural representan el 54,2% de la lista y tienen mayor importancia en las categorías H, R y B del sistema de clasificación Anatómica, Terapéutica y Química para sustancias farmacológicas y medicamentos. Además, se analizó la influencia de los productos naturales en las categorías con el mayor número de ingredientes activos J, L y N, así como también en la categoría C la cual está asociada a la principal causa de muerte en Ecuador.
Palabras clave: ingredientes activos, clasificación, metabolitos, salud pública, fuente.
Abstract: This paper presents the analysis of the origin of 391 active ingredients of the 9th list of essential medicines of Ecuador, using the system proposed by Newman and Cragg with certain modifications in order to highlight the contribution of natural products in the discovery and development of the drugs used in the Ecuadorian public health system. The active ingredients of natural origin represent 54.2% of the list and have greater importance in the categories H, R and B of the Anatomical, Therapeutic and Chemical classification system for pharmacological substances and medicines. In addition, the influence of natural products was analyzed in the categories with the highest number of active ingredients J, L and N, as well as in category C, which is associated with the main cause of death in Ecuador.
Keywords: active ingredients, classification, metabolites, public health, source.
INTRODUCTION
The use of natural products as lead compounds in the process of drug discovery has been revitalized[1]. This is due to three factors: 1) the technology based on synthetic molecules being less efficient than desired [2], 2) the evaluation of natural products in new biological targets [1], [3] 3) the great diversity of molecular scaffolds found in the living organisms [4]. Between 2003 and 2012, Newman and Cragg ([5]-[7]) categorized 1,355 drugs approved by the U.S. Food and Drug Administration (FDA) in the period of 1981-2010, according to their source. They pointed out the importance of natural products (NP) in the discovery and development of drugs, not only as lead compounds or as drugs properly, but as inspiration for the design of new ones. Furthermore, they identified that NP have influence mainly on the drugs used for treatment of cancer and infectious diseases. Following the methodology proposed by Newman and Cragg, Jones [8] analyzed the influence of NP on the World Health Organization Essential Medicines List, 13th Revision, which has nearly 300 drugs. In that work the 210 small-molecule therapeutic agents were classified, and among them 139 have a direct influence by NP. In addition, Tao [2] used the Newman and Cragg’s methodology to determine the NP- related drugs named by them as NP lead of drugs (NPLDs); in a list of 749 pre-2013 FDA approved and 263 clinical trial molecule drugs, these made up 442. Based on the previous information, Tao’s group [2] generated and evaluated distribution patterns in the chemical space represented by the molecular scaffold and fingerprints of 137,836 non-redundant natural products.
On the other hand, the Essential Medicines List (EML) is an inventory, which defines the most relevant drugs for a country, according to the prevalence and prevention of diseases in the population, drug efficiency, marketing, etc. In the case of Ecuador, the drugs of the Essential Medicines List are classified according to the ATC (Anatomical, Therapeutic, Chemical classification system) code established by the WHO [9]. However, none ofthe drugs on the Ecuadorian Essential Medicines List (EEML) have been classified according to their origin. In this work, the origin of drugs on the EEML was determined using Newman and Cragg methodology to evaluate the contribution of NP in each category of ATC code. Besides, the origin of some drugs was described with the goal of highlighting the contribution of natural products on the discovery and development of the drugs used for the treatment of prevalent diseases in Ecuador.
MATERIALS AND METHODS
The active ingredients (AIs) of the 9th Ecuadorian Essential Medicines List [10] were classified according to the categories proposed by Newman and Cragg [7]. The category “M” was added to gather the inorganic compounds. In addition, the category B was divided into two, following the criteria of Tao’s group [2].
Categories
“B*” Human biologics include peptides, nucleic acids, proteins, and antibodies.
“B” Nonhuman biologics include peptides, nucleic acids, proteins, and antibodies.
“M” Minerals.
“N” Natural product.
“NB” Natural product “Botanical”.
“ND” Derived from a natural product and is usually a semisynthetic modification.
“S”Totally synthetic drug, often found by random screening/modification of an existing agent.
“S*” Made by total synthesis, but the pharmacophore is/ was from a natural product.
“V” Vaccine.
Subcategory: “NM” Natural product mimic.
First, a search of the compounds previously classified was carried out in the books and articles that follow:
D. J. Newman and G. M. Cragg, “Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010” J. Nat. Prod., vol. 75, no. 3, pp. 311-335, 2012.
L. Tao et al., “Clustered Distribution of Natural Product Leads of Drugs in the Chemical Space as Influenced by the Privileged Target-Sites,” Sci. Rep., vol. 5, 2015.
W. P. Jones, Y. Chin, and A. D. Kinghorn, “The role of pharmacognosy in modern medicine and pharmacy,” Curr. Drug Targets, vol. 7, no. 3, pp. 247-264, 2006.
R. S. Vardanyan and V. J. Hruby, Synthesis of Essential Drugs, First. Amsterdam, The Netherlands: Elsevier B.V., 2006.
T. L. Lemke, D. A. Williams, V. F. Roche, and W. Zito, Foye’s Principles of Medicinal Chemistry, 6th ed., Philadelphia: Lippincott Williams & Wilkins, 2008.
W. Sneader. Drug Discovery A History. Chichester. John Wiley & Sons Ltd., 2005.
The AIs not found in these references were assigned according to the information found in scientific articles or web pages. The list of all AIs with their corresponding references is described in Supporting Material 1.
RESULTS AND DISCUSSION
391 AIs were determined in the 9th Ecuadorian Essential Medicines List [10]. Ortiz-Prado [9] determined 397 AIs in 533 pharmaceutical forms and 725 different concentrations. This discrepancy could be attributed to the fact that in this work, products like rehydration salts, multivitamins and vitamin combinations were not considered as AIs. Nevertheless, the components of these mixtures were considered, such as vitamin A, ascorbic acid, and sodium chloride. Trace elements, Zinc products, and hypertonic solutions were considered as one AI each and categorized in the group M. In addition, the multi-enzymes and gelatin agents were considered as Ais and assigned to the categories B* and B, respectively. In the product electrolytes with carbohydrates, only dextrose was considered and assigned to the category N. Also, for the product Ringer lactate, only the component sodium lactate was considered as AI and assigned to the category ND. Each vaccine was considered as AI.
The determination of the AIs’ sources is summarized in Figure 1. For all 391 Ais, there are two principal sources with 108 (27.6%) and 96 (24.6%) corresponding to the groups S and ND, respectively. The third group in importance is N (69, 17.6%). These three groups have AIs in all categories of ATC code. Considering all synthetic-type groups (included the minerals) as only one and the others grouped as natural-type, 179 (45.8%) AIs occupy the non-natural category. It is evident that the majority of AIs of EEML have a natural origin. To evaluate the contribution of natural products in EEML more precisely, only the small molecules were considered. In other words, the EEML was analyzed without the groups V, M, B and B* due to its high molecular weight or to its inorganic origin. Overall, 324 AIs were considered as small molecules. The natural-type ones (165, 50.9%) have a slightly higher contribution than synthetic-ones (159, 49,1%) (Figure 2). These results are in accordance with those reported by Newman and Cragg [7].
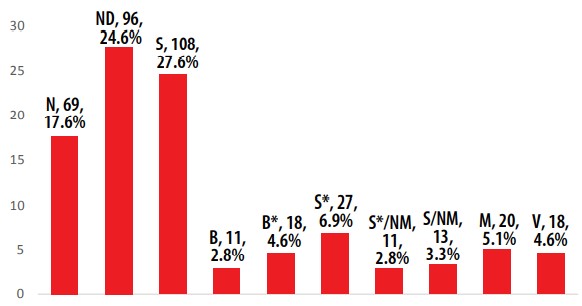
Figure 1
AIs in the EEML
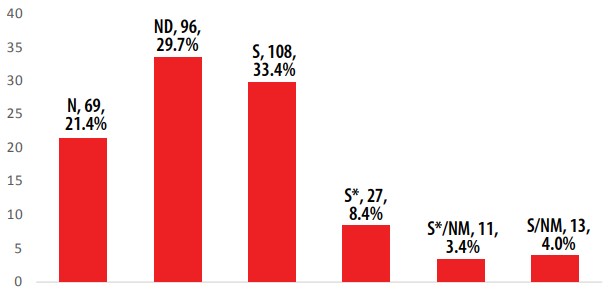
Figure 2
Source of small molecules in EEML
The origin of AIs of EEML can be analyzed by each group of the ATC code. It is important to consider that some AIs are used for more than one treatment in the ATC code (15 are repeated twice and 2 three times). For this reason, the total number of AIs used increased from 391 to 412. The results are summarized in the Tables 1 and 2.
| ATC code | N | S | ND | B | B* | S* | S*/ NM | S/ NM | M | V | Total |
| A | 10 | 5 | 6 | 0 | 3 | 2 | 1 | 0 | 7 | 0 | 34 |
| B | 10 | 1 | 4 | 6 | 3 | 0 | 0 | 1 | 11 | 0 | 36 |
| C | 5 | 13 | 9 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 30 |
| D | 6 | 4 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 15 |
| G | 4 | 3 | 6 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 16 |
| H | 3 | 2 | 7 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 14 |
| J | 12 | 19 | 26 | 0 | 4 | 9 | 4 | 1 | 0 | 18 | 93 |
| L | 11 | 12 | 13 | 7 | 6 | 9 | 3 | 1 | 0 | 0 | 62 |
| M | 1 | 7 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 13 |
| N | 4 | 27 | 9 | 0 | 0 | 2 | 1 | 3 | 1 | 0 | 47 |
| P | 2 | 8 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 13 |
| R | 3 | 2 | 6 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 13 |
| S | 3 | 4 | 5 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 13 |
| V | 2 | 6 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 13 |
Distribution of AIs in the EEML by group of ATC code
A=Alimentary tract and metabolism; B=Blood and blood forming organs; C=Cardiovascular system; D=Dermatological drugs; G=Genitourinary system and reproductive hormones; H=Systemic hormonal preparations, excluding reproductive hormones and insulins; J=Antiinfectives for systemic use; L=Antineoplastic and immunomodulating agents; M= Musculoskeletal system; N=Nervous system; P=Antiparasitic products, insecticides and repellents; R=Respiratory system; S=Sensory organs; V=Various ATC structures.
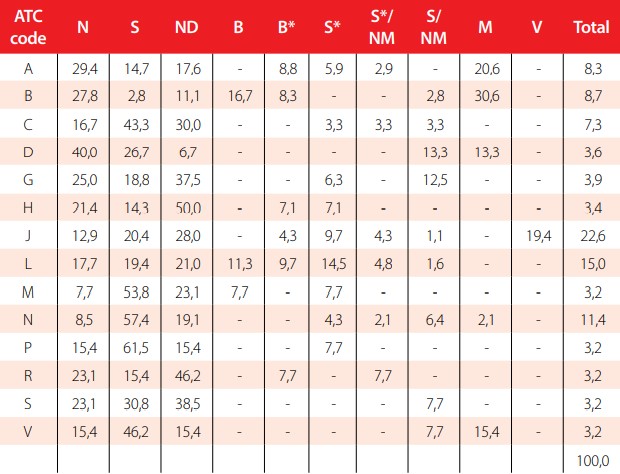
Distribution of AIs in the EEML by group of ATC code in percentages
A=Alimentary tract and metabolism; B=Blood and blood forming organs; C=Cardiovascular system; D=Dermatological drugs; G=Genitourinary system and reproductive hormones; H=Systemic hormonal preparations, excluding reproductive hormones and insulins; J=Antiinfectives for systemic use; L=Antineoplastic and immunomodulating agents; M= Musculoskeletal system; N=Nervous system; P=Antiparasitic products, insecticides and repellents; R=Respiratory system; S=Sensory organs; V=Various ATC structures.For a clearer notion concerning the source of AIs in the groups of ATC code, two principal categories were set: the natural-type (N, ND, B, B* and V) and the synthetictype (S, S*, S/NM, S*/NM and M). Figure 3 shows the differences between the mean value of overall EEML and the number of AIs inside of each group or ATC code. Also, the analysis only for small molecules was made (Figure 4), where the positive values mean a major natural contribution while the negative values a major synthetic contribution. The categorization of these groups as natural or synthetic-type does not mean that within the group there are not important natural-type AIs or vice versa. In addition, the structure of the small molecules can be envisioned according to the classification by origin and ATC’s category in Supporting Material 2.
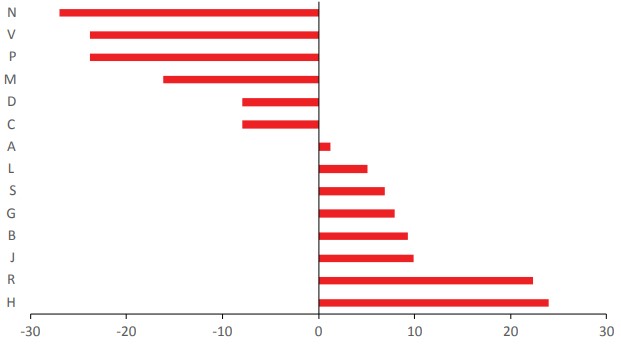
Figure 3.
Contribution of the source (natural-type or synthetic-type) to the AIs of EEML in the groups of ATC code compared with a mean value.
In the EENL, the group of the ATC code with majority of AIs is the J group (93, 22,6%) (Tables 1 and 2). This group is related to anti-infective drugs for systemic use. It is important to recognize the significance of the vaccines for this group. Basically, it is the only group where the category V appears (Table 1). The main category within this group is the ND. Only the category M is not present in this group. When strictly considering the synthetic drugs assigned to the S category, it was observed that 20.2% of AIs in this group do not have any “natural” influence (Table 2). Moreover, when only the small molecules are considered, the J group has a slightly larger synthetic-type component compared to the mean value (Figure 4).
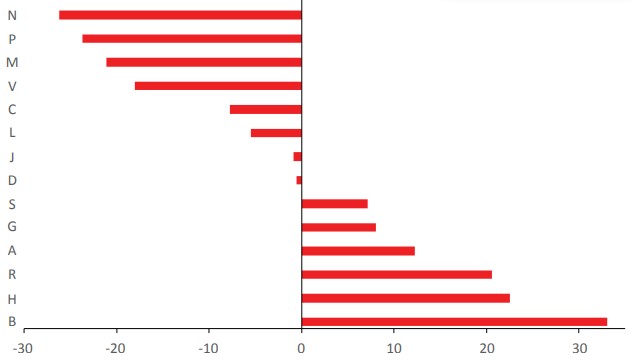
Figure 4
Contribution of the source (natural-type or synthetic-type) to the small molecules of EEML in the groups of ATC code compared with a mean value.
For visualizing the impact of the origin of AIs in infectious diseases, bronchitis, one of the lower respiratory infections, was considered. These infections have been recognized as the main cause of death [11] regional, and national health policies. In the Global Burden of Disease Study 2013 (GBD 2013 during the period of 1990-2013 in Ecuador. The drugs in EEML for the treatment of bronchitis are: Ampicillin (ND), Cotrimoxazole, which have two AIs Sulfamethoxazole (S) and Trimethoprim (S*), Clarithromycin (ND) and Levofloxacin (S*). Only one of these AIs has a totally synthetic origin—Sulfamethoxazole. The Ampicillin is synthetized from (+)-6-aminopenicillanic acid, which is produced from a brew of the Penicillium mold [12]. Clarithromycin is a macrolide antibiotic which was obtained for the first time in 1990 (Morimoto, et al., 1990) from a derivative of Erythromycin synthetized by Flyn [13]. The Erythromycin is an antibiotic which was isolated in 1952 from a strain of Streptomyces erythreus, present in a soil sample from the Philippines [13]. This short example is useful to remark that most antibiotic drugs have an origin in microorganisms. Also, it helps to target most antibiotics with new molecular skeletons fight against the resistant strains, which is a serious public health problem [14]. It is envisioned that a bigger effort should be made in the screening of secondary metabolites from microorganisms, especially in areas of rich biological diversity which exist in tropical countries like Ecuador. On the other hand, Trimethoprim has a synthetic origin and is a strong dihydrofolate reductase inhibitor. Trimethoprim has a diaminopyrimidine fragment in its structure. This structural factor is similar to the pteridine ring of folic acid [15]. The activity of this drug could be attributed to the high affinity of the diaminopyrimidine fragment with the bacterial dihydrofolate reductases. Therefore, it can be intuited that Trimethoprim was inspired by folic acid. Levofloxacim is an antibiotic drug of the fluoroquinolones group with a synthetic origin. All quinolones have their origin in the nalixidic acid, a by-product in the synthesis of natural product chloroquine [16]. Therefore, quinolones were inspired by quinine. Quinine was isolated from Cinchona officialis, a native tree from South America. This plant was intensely exploited during the 17th and 18th centuries in Loja, Ecuador because it was one of the few remedies for malaria at that time. The use of this plant was common between the natives before Europeans arrived to the New World [17].
The second largest group of AIs is the L group (62, 15.0%) related to antineoplastic and immunomodulation agents. ND is the main category within this group. It is remarkable that in this group only the categories M and V are not present. The relevance of the AIs of category B* in this group is greater than in other groups. This group is mostly composed of natural-type AIs (Figure 4). Newman and Cragg [7] found that most anti-cancer drugs approved by the FDA in the period of 1940-2010 had natural-type origin. According to official data [18], neoplastic causes were the second leading cause of deaths in Ecuador in 2016. The deadliest kind of neoplastic illness was stomach cancer, around 17.2% of overall neoplastic illnesses. According to EEML, the AIs used in the treatment of this kind of cancer are Docetaxel (ND) and Mitomycin (N). Docetaxel is obtained by the semi-synthesis of 10-deacetylbaccatine III, commonly known as 10-DAB, from the ubiquitous Taxus baccata, or English yew. It is a well-known case about the importance of pharmacognosy in the discovery and development of new drugs. Two facts are remarkable: The first one is the discovery of paclitaxel from the extracts of the bark of the Pacific yew (Taxus brevifolia). This compound showed great anti-tumor activity for some types of cancer [19]. The main issue for using paclitaxel as a therapeutic agent lies on the fact that the old yews were the unique source of this drug. The harvesting of the bark kills the tree. The age for the maximum yield by tree was too high to consider this idea as practical. The complex structure of paclitaxel represents a big challenge for synthetic chemists. But, the solution was found in Taxus baccata. The molecule 10-DAB (the most complex residue of paclitaxel) is produced in the needles of this yew. Using 10-DAB as starting material, the efficient semi-synthesis was achieved by the scientists of Bristol-Myers Squibb Company in 1994 [20]
The third largest group of AIs is the N group (47, 11.4%), which is related to nervous system drugs. This group has a great synthetic component, more than all other groups. However, there is an AI from natural source with great importance to treat the deadliest disease of this group in Ecuador, which is Alzheimer’s disease [18]. This compound is Galanthamine. The isolation and evaluation of the biological activity of this AI was inspired by ethnomedical reports [21]. Galanthamine was isolated from Galanthus nivalis, a specie from the Amaryllidaceae family which is richly represented in the tropics and has pronounced centers of diversity in South Africa and the Andean region. Some genera are also found in the Mediterranean area and temperate regions of Asia. In addition, in Iberoamerica there is vigorous research about the determination of the ability of the Amaryllidaceae species to produce Galanthamine in good yield to satisfy the market necessities [22].
In 2016 the main cause of death in Ecuador was associated with the cardiovascular system [18], group C according to the ATC code. This group is mainly composed of synthetic-type AIs. However, the natural-type AIs are relevant in this group. Simvastatin is a compound used to lower cholesterol levels. It was obtained by the synthetic modification from lovastatin, which was isolated from the fungus strain of Aspergillus terreus [23]. The discovery of statins in fungi and their mechanism of action (inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, or HMG-CoA) constitute a clear example of drug research and development. In addition, another relevant and classic example is Digitoxin, a natural compound isolated from Digitalis purpurea.
A remarkable example (which is not present in the EEML) of a natural source of a medicine found in Ecuador is the FulyzaqTM. The AI is Crofelemer, a purified oligomeric proanthocyanidin from the latex of Croton lechleri Müll.Arg. locally known as “Dragon’s blood” and used in the treatment of wounds [24]. This botanical drug has been approved by the FDA as the first anti-diarrheal drug for HIV/AIDS patients [25]. In the author’s opinion, the inclusion of botanical drugs in the market means an opportunity for research in the search for active ingredients of drugs in countries with great biodiversity such as Ecuador. As it has been pointed out, the role of natural products as sources of medicine is indispensable. Either by its structure, by its activity, or by serendipity, the research of natural products is of great importance in drug discovery and development.
CONCLUSIONS
The EEML has been categorized according to the drug origin. The natural-type AIs were found to be slightly more abundant and to have a greater influence on the H, R and B categories of the ATC code, even when the small-molecule criterion was considered. In addition, the group M was created to group AIs of mineral or inorganic origin. This work is an example of the contribution of natural products on the public health of a nation.
ACKNOWLEDGMENTS
To the Universidad Regional Amazónica Ikiam for supporting this work.
AUTHORS’ CONTIBUTIONS
In the framework of the project "I+P+I: Investigación de Excelencia, Posgrado e Igualdad” financed by European Union through the Agencia Española de Desarrollo Español (AECID), under supervision of the expedient No. 2018/SPE/0000400194
CONFLICTS OF INTERESTS
All authors declare that they have no conflicts of interest.
References
Carter, G. T. (2011). Natural products and Pharma 2011: Strategic changes spur new opportunities. Natural Product Reports, 28(11), 1783-1789. doi: https://doi.org/10.1039/c1np00033k.
Tao, L., Zhu, F., Qin, C, Zhang, C, Chen, S., Zhang, P., .. Chen, Y. Z. (2015). Clustered Distribution of Natural Product Leads of Drugs in the Chemical Space as Influenced by the Privileged Target-Sites. Scientific Reports, 5. doi: https://doi.org/10.1038/srep09325.
Cragg, G. M., Grothaus, P. G., & Newman, D. J. (2014). New horizons for old drugs and drug leads. Journal of Natural Products, 77(3), 703-723. doi: https://doi.org/10.1021/np5000796.
Buss, Antohy D. and Buttler, M. S. (Eds.). (2009). Natural Product Chemistry for Drug Discovery (First). Cambridge, England: Royal Society of Chemistry. doi: https://doi.org/10.1039/9781847559890.
Newman, D.J, Cragg, G., & Snader, K. (2003). Natural Products as sources of New Drugs over the Period 1981 - 2002. Journal of Natural Products, 66(7), 1022-1037. doi: https://doi.org/10.1021/np200906s.
Newman, David J, Cragg, G. M., Newman, D. J., & Cragg, G. M. (2007). Natural Products as Sources of New Drugs over the Last 25 Years. Journal of Natural Products, 70(February), 461-477. doi: https://doi.org/10.1021/np068054v.
Newman, D J, & Cragg, G. M. (2012). Natural Products as Sources of New Drugs over the 30 Years. Journal of Natural Products, 75(3), 311-335. doi: https://doi.org/10.1021/np200906s.
Jones, W. P., Chin, Y., & Kinghorn, A. D. (2006). The role of pharmacognosy in modern medicine and pharmacy. Current Drug Targets, 7(3), 247-264. doi: https://doi.org/10.2174/138945006776054915.
Ortiz-Prado, E., Galarza, C., León, F. C., & Ponce, J. (2014). Acceso a medicamentos y situación del mercado farmacéutico en Ecuador, Revista Panamericana de Salud Pública, 36(1), 57-62. Retrieved from https://www.scielosp.org/article/rpsp/2014.v36n1/57-62/es/#:~:text=En%20Ecuador%2C%20del%20total%20de,solo%2010%25%20a%20nivel%20rural.
Consejo Nacional de Salud. (2014). Cuadro Nacional de Medicamentos Básicos y Registro Terapéutico [PDF archive]. Retrieved from http://apps.who.int/medicinedocs/documents/s19429es/s19429es.pdf
Naghavi, M., Wang, H., Lozano, R., Davis, A., Liang, X., Zhou, M., . Temesgen, A. M. (2015). Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet, 385(9963), 117-171. doi: https://doi.org/10.1016/S0140-6736(14)61682-2
Batchelor, F. R., Doyle, F. P., Naylet, J. H. C., & Rolison, G. N. (1959). Synthesis of Penicillin: 6-Aminopenicillanic Acid in Penicillin Fermentations. Nature, 183, 257. doi: http://dx.doi.org/10.1038/183257b0
Mcguire, J. M., Bunch, R. L., Anderson, R. C., Boaz, H. E., Flynn, E. H., Powell, H. M., & Smith, J. W. (1952). Ilotycin, a new antibiotic. Antibiotics & Chemotherapy(Northfield, Ill.), 2(6), 281—283. http://europepmc.org/abstract/MED/24541924
Amann, S., Neef, K., & Kohl, S. (2019). Antimicrobial resistance (AMR). European Journal of Hospital Pharmacy, 26(3), 175-177.
Vardanyan, R. S., & Hruby, V. J. (2006). Synthesis of Essential Drugs (First). Amsterdam, The Netherlands: Elsevier B.V. doi: http://dx.doi.org/10.1016/B978-044452166-8/50003-0
[16] Emmerson, A. M., & Jones, A. M. (2003b). The quinolones: decades of development and use. Journal of Antimicrobial Chemotherapy, 51(suppl_1), 13-20. doi: https://doi.org/10.1093/jac/dkg208
Kaufman, T. S., & Rúveda, E. A. (2005). The quest for quinine: Those who won the battles and those who won the war. Angewandte Chemie - International Edition, 44(6), 854-885. doi: https://doi.org/10.1002/anie.200400663
Instituto Nacional de Estadísticas y Censos. (2016). Estadísticas Vitales [PDF archive]. Retrieved from https://www.ecuadorencifras.gob.ec/documentos/web-inec/Poblacion_y_Demografia/Nacimientos_Defunciones/2016/Presentacion_Nacimientos_y_Defunciones_2016.pdf
Wani, M. C., Taylor, H. L., Wall, M. E., Coggon, P., & McPhail, A. T. (1971). Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. Journal of the American Chemical Society, 93(9), 2325-2327. doi: https://doi.org/10.1021/ja00738a045
Poss, M. A., Moniiot, J. L., Trifunovich, I. D., Kucera, D. J., Thottathil, J. K., Chen, S. H., & Wei, J. (1994) Novel sidechainbearing taxanes and intermediates thereof. W.O. patent 9119787. Retrieved from https://patents.google.com/patent/WO1994014787A1/en
Heinrich, M., & Teoh, H. L. (2004). Galanthamine from snowdrop — the development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. Journal of Ethnopharmacology, 92, 147-162. doi: https://doi.org/10.1016/j.jep.2004.02.012
CYTED. (2018). Detalles de la Red 416RT0511 La Biodiversidad Iberoamericana Como Fuente de Recursos Naturales Para Su Explotación Sostenible (BIFRENES). September 19, 2018. Retrieved from http://www.cyted.org/?q=es/detalle_proyecto&un=909
Endo, A. (2010). Review A historical perspective on the discovery of statins. The Proceedings of the Japan Academy, Series B, 86, 484-493. doi: https://doi.org/10.2183/pjab.86.484
Namjoyan, F., Kiashi, F., Moosavi, Z. B., Saffari, F., & Makhmalzadeh, B. S. (2016). Efficacy of Dragon’s blood cream on wound healing: A randomized, double-blind, placebo-controlled clinical trial. Journal of Traditional and Complementary Medicine, 6(1), 37-40. doi: https://doi.org/10.1016/j.jtcme.2014.11.029
Georgiev, M. I. (2014). Natural products utilization. Phytochemistry Reviews, 13, 339-341. doi: https://doi.org/10.1007/s11101-014-9363-3